Content Menu
● Introduction to Tungsten Carbide
>> Chemical Structure
● Synthesis of Tungsten Carbide
● Chemical Properties and Reactivity
● Applications of Tungsten Carbide
● Advanced Applications
● Challenges and Future Directions
● Environmental Impact
● Conclusion
● FAQs
>> 1. What is Tungsten Carbide?
>> 2. How is Tungsten Carbide Synthesized?
>> 3. Is Tungsten Carbide Reactive to Acids?
>> 4. What are the Primary Applications of Tungsten Carbide?
>> 5. Can Tungsten Carbide be Recycled?
● Citations:
Tungsten carbide is a compound made from tungsten and carbon, renowned for its exceptional hardness, wear resistance, and durability. It is widely used in industrial applications, including cutting tools, drill bits, and wear-resistant components. However, understanding its reactivity is crucial for its safe and effective use. In this article, we will delve into the reactivity of tungsten carbide, exploring its chemical properties, synthesis methods, and applications.

Introduction to Tungsten Carbide
Tungsten carbide is a dense, metal-like substance with a light gray color and a bluish tinge. It is prepared by heating powdered tungsten with carbon black in the presence of hydrogen at high temperatures (1,400°C to 1,600°C). The resulting compound is extremely hard, ranking about 9.0 to 9.5 on the Mohs scale, making it one of the hardest materials available.
Chemical Structure
Tungsten carbide forms a hexagonal crystal structure, which contributes to its hardness and stability. The compound is primarily composed of tungsten and carbon in a 1:1 ratio, often mixed with a metallic binder like cobalt to enhance its toughness and durability.
Synthesis of Tungsten Carbide
The synthesis of tungsten carbide involves several methods:
1. High-Temperature Reaction: Tungsten metal or powder is reacted with carbon at temperatures between 1,400°C and 2,000°C.
2. Fluid Bed Process: This method uses a lower temperature (900°C to 1,200°C) and involves reacting tungsten metal or blue tungsten oxide (WO3) with a CO/CO2 gas mixture and hydrogen.
3. Chemical Vapor Deposition (CVD): Tungsten hexachloride reacts with hydrogen and methane at 670°C to form tungsten carbide.
WCl6 + 6H2 + CH4 → WC + 6HC
Chemical Properties and Reactivity
Tungsten carbide is highly stable and resistant to corrosion at normal temperatures. However, it reacts with certain substances under specific conditions:
- Oxidation: Tungsten carbide starts to oxidize at around 500°C to 600°C, forming tungsten trioxide (WO3) when heated in an oxygen-containing atmosphere.
- Acid Resistance: It is resistant to most acids but dissolves in a mixture of nitric acid and hydrofluoric acid.
WC + HNO3/HF → Dissolves
- Halogen Reactions: Tungsten carbide reacts with fluorine at room temperature and with chlorine above 400°C.
Applications of Tungsten Carbide
Due to its exceptional hardness and wear resistance, tungsten carbide is used in various applications:
1. Cutting Tools and Drill Bits: Its ability to maintain sharpness under high-stress conditions makes it ideal for cutting tools and drill bits.
2. Jewelry: Tungsten carbide is also used in jewelry due to its durability and aesthetic appeal.
3. Industrial Machinery: Its high thermal stability and resistance to wear make it suitable for components in industrial machinery.
4. Automotive and Aerospace: Tungsten carbide is used in these sectors for its high strength and resistance to wear, contributing to the longevity of components.
5. Medical Tools: It is used in surgical instruments for its hardness and resistance to corrosion.
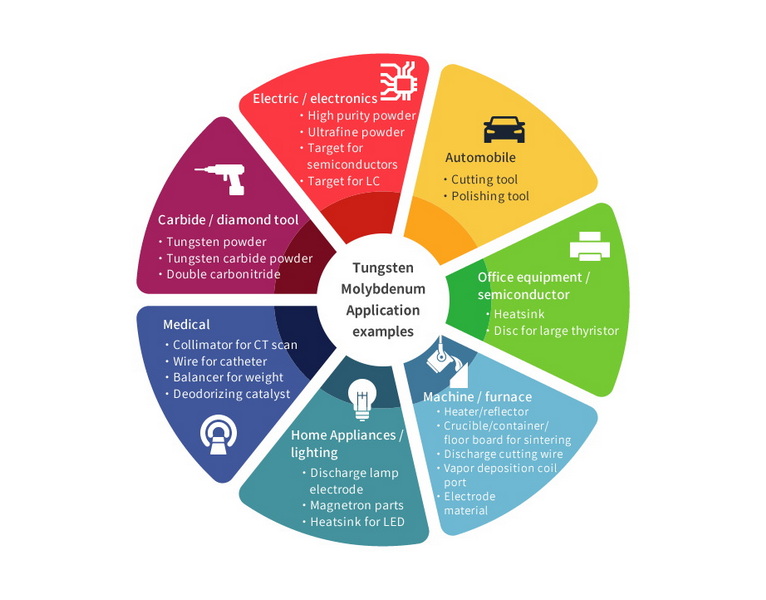
Advanced Applications
In recent years, tungsten carbide has seen advancements in its applications:
- Catalysis: Tungsten carbide is being explored as a catalyst in hydrogenation reactions due to its platinum-like properties, offering a cost-effective alternative for biomass upgrading[7].
- Energy Storage: Research into tungsten carbide-based materials for energy storage devices is ongoing, leveraging its high conductivity and stability.
Challenges and Future Directions
Despite its many advantages, tungsten carbide faces challenges such as brittleness and the need for a binder to enhance toughness. Future research aims to improve its synthesis conditions and explore new applications in emerging fields like renewable energy and advanced materials.
Environmental Impact
The recycling of tungsten carbide is crucial to reduce waste and conserve resources. Techniques for reclaiming and reusing worn-out tools are being developed to minimize environmental impact.
Conclusion
Tungsten carbide is a highly stable compound with exceptional hardness and wear resistance. While it is generally non-reactive at normal temperatures, it can react with certain substances under specific conditions. Understanding its chemical properties and reactivity is crucial for its effective use in various industrial and consumer applications.

FAQs
1. What is Tungsten Carbide?
Tungsten carbide is a compound made from tungsten and carbon, known for its hardness and wear resistance. It is used in cutting tools, drill bits, and other wear-resistant components.
2. How is Tungsten Carbide Synthesized?
Tungsten carbide is synthesized by reacting tungsten with carbon at high temperatures or through chemical vapor deposition methods.
3. Is Tungsten Carbide Reactive to Acids?
Tungsten carbide is resistant to most acids but dissolves in a mixture of nitric acid and hydrofluoric acid.
4. What are the Primary Applications of Tungsten Carbide?
Tungsten carbide is primarily used in cutting tools, drill bits, industrial machinery components, jewelry, and medical tools due to its hardness and durability.
5. Can Tungsten Carbide be Recycled?
Yes, tungsten carbide can be recycled. Worn-out tools and scrap material can be reclaimed and reused, reducing waste and conserving resources.
Citations:
[1] https://www.science.gov/topicpages/t/tungsten+carbide+leaching.html
[2] https://www.refractorymetal.org/tungsten-carbide-uses-properties.html
[3] https://www.linkedin.com/pulse/applications-tungsten-carbide-zzbettercarbide
[4] https://www.alamy.com/stock-photo/tungsten-carbide.html
[5] https://en.wikipedia.org/wiki/Tungsten_carbide
[6] https://create.vista.com/photos/tungsten-carbide/
[7] https://www.ch.nat.tum.de/en/ac4/research-topics/tungsten-carbide/
[8] https://www.retopz.com/understanding-the-chemical-properties-of-tungsten-carbide-an-explanatory-overview/
[9] https://www.gettyimages.hk/%E5%9C%96%E7%89%87/tungsten-carbide?page=2
[10] https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5174366.htm
[11] https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/celc.202300722
[12] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[13] https://www.mdpi.com/1420-3049/30/1/84
[14] https://www.sollex.se/en/blog/post/tungsten-carbide-and-technology-part-2
[15] https://pubs.acs.org/doi/10.1021/acsomega.8b03449
[16] https://www.azom.com/article.aspx?ArticleID=1203
[17] https://www.nature.com/articles/s41467-018-03429-z
[18] https://www.carbideprobes.com/wp-content/uploads/2019/07/TungstenCarbideDataSheet.pdf
[19] https://www.samaterials.com/content/application-of-tungsten-in-modern-industry.html
[20] https://www.imetra.com/tungsten-carbide-material-properties/
[21] https://www.azom.com/properties.aspx?ArticleID=1203
[22] https://www.freepik.com/free-photos-vectors/tungsten
[23] https://www.istockphoto.com/photos/tungsten-carbide
[24] https://stock.adobe.com/search?k=tungsten+carbide
[25] https://www.gettyimages.hk/%E5%9C%96%E7%89%87/tungsten-carbide
[26] https://www.shutterstock.com/search/tungsten
[27] https://cen.acs.org/materials/Chemistry-Pictures-Tungsten-carbide-slice/103/web/2025/02
[28] https://stock.adobe.com/search?k=carbide
[29] https://pubchem.ncbi.nlm.nih.gov/compound/Tungsten-carbide
[30] https://www.freepik.com/free-photos-vectors/tungsten-carbide
[31] http://www.tungsten-carbide.com.cn
[32] https://www.istockphoto.com/photos/tungsten-carbide-drill-bits
[33] https://www.britannica.com/science/tungsten-chemical-element
[34] https://www.shutterstock.com/search/tungsten-carbide
[35] https://www.lenntech.com/periodic/elements/w.htm
[36] https://www.hyperionmt.com/en/Resources/materials/cemented-carbide/thermal-properties/
[37] https://www.linkedin.com/pulse/properties-tungsten-carbide-shijin-lei-1c
[38] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[39] https://www.vedantu.com/chemistry/tungsten-carbide
















