Content Menu
● Introduction to Calcium Carbide
● Raw Materials for Calcium Carbide Production
● The Chemical Reaction
● Industrial Production Process
>> 1. Preparation of Raw Materials
>> 2. Furnace Types
>> 3. Electrothermic Reduction
>> 4. Tapping and Cooling
>> 5. Crushing and Screening
>> 6. Impurity Removal
>> 7. Packaging and Storage
● Safety and Environmental Controls
● Applications of Calcium Carbide
● Historical Development of Calcium Carbide Production
● Environmental Impact and Sustainability Efforts
● Technical Details on Furnace Operation and Control
● Recent Technological Advancements and Future Trends
● Conclusion
● FAQ
>> 1. What raw materials are used in the production of calcium carbide?
>> 2. Why is an electric arc furnace used for calcium carbide production?
>> 3. How is safety managed during calcium carbide production?
>> 4. What are the main industrial applications of calcium carbide?
>> 5. How is the quality of calcium carbide assessed?
● Citations:
Calcium carbide (CaC₂) is a vital industrial chemical used extensively in the production of acetylene gas, steelmaking, fertilizers, and various other applications. As a high-tech enterprise specializing in the research, production, and sales of carbide products, understanding the manufacturing process of calcium carbide is essential for optimizing quality, safety, and efficiency. This article provides a comprehensive overview of the industrial production of calcium carbide, detailing raw materials, furnace types, production steps, safety measures, and applications, supplemented with illustrative figures to clarify the process.
Introduction to Calcium Carbide
Calcium carbide is a chemical compound primarily composed of calcium and carbon. It appears as grey or brown lumps in technical-grade form, containing approximately 80-85% CaC₂, with the remainder being impurities such as calcium oxide (CaO), calcium phosphide (Ca₃P₂), calcium sulfide (CaS), and others. The compound reacts with water to produce acetylene gas (C₂H₂), which has widespread industrial uses.
Raw Materials for Calcium Carbide Production
The main raw materials for calcium carbide production are:
- Lime (Calcium Oxide, CaO): Derived from limestone (CaCO₃) by calcination in a lime kiln.
- Carbon Source: Usually coke, anthracite coal, or a mixture of both.
The quality and particle size of these raw materials significantly affect furnace performance and product quality. Typically, raw materials are screened to sizes between 5 mm and 40 mm to ensure proper porosity and electrical conductivity in the furnace charge.
The Chemical Reaction
The fundamental chemical reaction in calcium carbide production is:
CaO+3C→CaC2+CO
This reaction is highly endothermic, requiring temperatures above 1600 °C to proceed efficiently, with industrial processes typically operating between 1800 °C and 2100 °C.
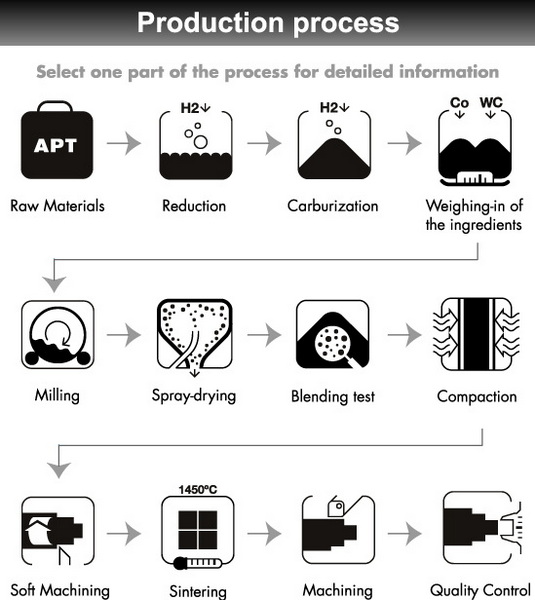
Industrial Production Process
1. Preparation of Raw Materials
- Drying: Moisture is removed from coke in a coke dryer.
- Calcination: Limestone is converted to lime (CaO) in a lime kiln.
- Screening and Mixing: The lime and coke are screened for size and mixed in the correct ratio, usually close to 1:3 (CaO to C) by mole.
2. Furnace Types
Calcium carbide is produced mainly in electric arc furnaces, which provide the necessary high temperatures through electrical energy. There are three basic types:
- Open Furnace: Carbon monoxide burns to CO₂ in contact with air above the charge.
- Closed Furnace: Gases are collected and reused or flared.
- Semi-covered Furnace: Mix is fed around electrode openings to create seals.
The electric arc furnace is the primary equipment used, with graphite electrodes lowered into the charge to generate arcs that melt and react the materials.
3. Electrothermic Reduction
Inside the furnace, the reaction proceeds in two steps:
- Calcination:
CaO+C→Ca+CO
- Carbide Formation:
Ca+2C→CaC2
The molten calcium carbide is tapped continuously from the furnace into cooled ladles, where it solidifies.
4. Tapping and Cooling
The molten calcium carbide is tapped from the furnace at temperatures between 1700 °C and 2100 °C. It is poured into molds or chills to solidify rapidly. Due to the high melting point and rapid cooling, the carbide solidifies quickly, requiring continuous maintenance of the tapping hole to prevent blockages or hazards.
5. Crushing and Screening
Once solidified, calcium carbide is crushed using jaw crushers and screened to the desired particle size. Crushing is often performed in an inert atmosphere or air-swept environment to prevent explosions caused by acetylene gas released upon contact with moisture.
6. Impurity Removal
Impurities such as ferrosilicon and iron-based materials are removed using high-powered electromagnets and manual sorting, improving product purity and performance.
7. Packaging and Storage
Calcium carbide is packaged in airtight containers, often with nitrogen filling to displace air and prevent moisture ingress. Storage facilities maintain strict temperature and humidity controls and fire and water protection to ensure product stability and safety.
Safety and Environmental Controls
- Dust Control: Fully enclosed dust collection systems are used during crushing, screening, and packaging to protect workers and reduce environmental emissions.
- Fume Extraction: Emissions of particulate matter, sulfur oxides, CO, and hydrocarbons are controlled with fabric filters, wet scrubbers, and ventilation systems.
- Operator Protection: Operators wear fireproof clothing, protective visors, and dark glasses during tapping to shield from hot gases and carbide spurts.
Applications of Calcium Carbide
- Acetylene Production: The primary use, where calcium carbide reacts with water to produce acetylene gas for welding, lighting, and chemical synthesis.
- Steelmaking: Used for desulfurizing iron and removing impurities during steel production.
- Fertilizers: Production of calcium cyanamide, a nitrogen fertilizer, via reaction with nitrogen gas.
- Other Uses: Pharmaceuticals, water treatment, construction materials, and historically, carbide lamps.
Historical Development of Calcium Carbide Production
The discovery of calcium carbide dates back to the late 19th century, with the first industrial production beginning in the early 1900s. The invention of the electric arc furnace by Paul Héroult in 1900 revolutionized the production process, enabling the high temperatures required for the reaction. Over the decades, improvements in furnace design, raw material processing, and safety protocols have significantly enhanced production efficiency and product quality. Today, calcium carbide production is a global industry, with major producers in China, the United States, and Europe.
Environmental Impact and Sustainability Efforts
Calcium carbide production is energy-intensive and generates emissions such as carbon monoxide and particulate matter. Modern plants implement advanced environmental controls, including gas scrubbing systems and dust collectors, to minimize air pollution. Efforts to improve energy efficiency include waste heat recovery systems and the use of renewable energy sources where feasible. Research is ongoing into alternative production methods that could reduce carbon footprints and enhance sustainability.
Technical Details on Furnace Operation and Control
Maintaining optimal furnace conditions is critical for efficient calcium carbide production. Operators monitor electrical parameters such as voltage, current, and power consumption to ensure stable arc conditions. Temperature sensors and gas analyzers provide real-time data to adjust feed rates and electrode positions. Automated control systems have been increasingly adopted to optimize furnace performance, reduce energy consumption, and improve safety.
Recent Technological Advancements and Future Trends
Recent advancements include the development of more durable electrode materials, improved refractory linings, and enhanced automation technologies. Digitalization and Industry 4.0 concepts are being integrated into calcium carbide plants, enabling predictive maintenance and process optimization through data analytics. Future trends may involve the use of alternative carbon sources, such as biomass-derived carbon, and the exploration of electrochemical methods for carbide synthesis.
Conclusion
Industrial calcium carbide production is a complex, energy-intensive process that relies on the high-temperature reaction of lime and carbon in electric arc furnaces. The process demands precise control over raw material quality, furnace operation, and safety measures to produce high-purity calcium carbide efficiently. This compound remains indispensable in acetylene production, steelmaking, fertilizer manufacture, and other industrial applications. Advances in automation, safety, and environmental controls continue to improve production efficiency and reduce hazards. As a high-tech enterprise engaged in carbide product development and manufacturing, mastering these processes ensures delivery of superior calcium carbide products to diverse industrial sectors.

FAQ
1. What raw materials are used in the production of calcium carbide?
Calcium carbide is produced from lime (CaO), derived from limestone, and a carbon source such as coke or anthracite coal. These materials must be screened to appropriate sizes to ensure proper furnace operation.
2. Why is an electric arc furnace used for calcium carbide production?
The reaction to form calcium carbide requires extremely high temperatures (around 2000 °C), which cannot be achieved by conventional combustion. Electric arc furnaces provide the necessary heat via electric arcs between graphite electrodes and the charge material.
3. How is safety managed during calcium carbide production?
Safety measures include enclosed dust collection systems, fume extraction, protective clothing for operators, nitrogen filling in packaging to prevent moisture contact, and continuous monitoring of acetylene gas concentrations to prevent explosions.
4. What are the main industrial applications of calcium carbide?
Calcium carbide is primarily used to produce acetylene gas for welding and chemical synthesis, in steelmaking for desulfurization, and in fertilizer production as calcium cyanamide. It also has applications in pharmaceuticals, water treatment, and construction.
5. How is the quality of calcium carbide assessed?
Quality is measured by acetylene gas yield upon hydrolysis, particle size distribution, and impurity levels such as phosphine and hydrogen sulfide content. Strict testing ensures compliance with industrial standards.
Citations:
[1] https://www3.epa.gov/ttnchie1/ap42/ch11/final/c11s04.pdf
[2] https://chemcess.com/calcium-carbide-properties-production-and-uses/
[3] https://sathee.prutor.ai/article/chemistry/chemistry-calcium-carbide/
[4] https://www.wis-chemicalmaterial.com/news/cac2-67479087.html
[5] https://www.alamy.com/stock-photo/calcium-carbide.html
[6] https://www.slideshare.net/slideshow/manufacturing-of-calcium-carbidepdf/262369751
[7] https://www.tjtywh.com/common-faqs-about-calcium-carbide-10-key-questions-customers-care-about.html
[8] https://patents.google.com/patent/US4594236A/en
[9] https://www.vedantu.com/chemistry/calcium-carbide
[10] https://en.wikipedia.org/wiki/Calcium_carbide
[11] https://www.tjtywh.com/tywh-calcium-carbide-manufacturing-process.html
[12] https://www.tjtywh.com/frequently-asked-questions-about-calcium-carbide-procurement-how-to-avoid-quality-issues-and-supply-shortages.html
[13] https://www.youtube.com/watch?v=olAlOs0Er00
[14] https://www.pyrometallurgy.co.za/InfaconXIV/149-McCaffrey.pdf
[15] https://www.youtube.com/watch?v=BtfSHPBy9Bg
[16] https://www.tjtywh.com/a-step-by-step-guide-to-making-calcium-carbide-at-home.html
[17] https://www.tjtywh.com/a-the-benefits-of-pulverized-calcium-carbide-in-industrial-applications.html
[18] https://enterclimate.com/calcium-carbide-manufacturing-unit-setup
[19] https://byjus.com/chemistry/calcium-carbide/
[20] https://www.tjtywh.com/a-exploring-the-process-of-making-calcium-carbide-a-how-to-guide.html
[21] https://www.tjtywh.com/a-the-role-of-calcium-carbide-in-industrial-processes-and-applications.html
[22] https://www.niir.org/blog/wp-content/uploads/2021/11/Manufacturing-Business-of-Calcium-Carbide-Calcium-Acetylide.-Investment-Opportunities-in-Chemical-Industry.-1.pdf
[23] https://www.cargohandbook.com/Calcium_Carbide
[24] https://en.wikipedia.org/wiki/Calcium_carbide
[25] https://www.shutterstock.com/search/calcium-carbide
[26] https://www.shutterstock.com/search/calcium-carbure
[27] https://www.gettyimages.com/photos/calcium-carbide
[28] https://en.wikipedia.org/wiki/Electric_arc_furnace
[29] https://www.shutterstock.com/search/crushing-screening-plant
[30] https://www.carbidellc.com
[31] https://patents.google.com/patent/US4594236A/en
[32] https://www.made-in-china.com/products-search/hot-china-products/Calcium_Carbide_Furnace.html
[33] https://zhongjia.en.made-in-china.com/product/GxLRrAXwOPYd/China-Double-Teeth-Roller-Crusher-for-Crushing-Calcium-Carbide-in-Malaysia.html
[34] https://www.instagram.com/carbidecalcium/
[35] https://tianyuanweihong.en.made-in-china.com/product/IdAfVWxPutpR/China-Calcium-Carbide-for-Acetylene-Production-Plant.html
[36] https://camachem.com/pt/blog/post/frequently-asked-question-about-calcium-carbide
[37] https://www.eiga.eu/uploads/documents/DOC196.pdf
[38] https://sathee.prutor.ai/article/chemistry/chemistry-calcium-carbide/
[39] https://www.tjtywh.com/calcium-carbide-gas-volume-and-production-efficiency-how-to-increase-acetylene-gas-yield.html
[40] https://www.tjtywh.com/how-to-safely-transport-and-store-calcium-carbide.html
[41] https://www.vaia.com/en-us/textbooks/chemistry/introductory-chemistry-9-edition/chapter-9/problem-28-calcium-carbide-mathrmcac2-can-be-produced-in-an-/
[42] https://www.guidechem.com/question/what-are-the-secrets-of-calciu-id157110.html
[43] https://www.tjtywh.com/calcium-carbide-quality-control-how-to-ensure-high-quality-products.html
[44] https://camachem.com/ru/blog/post/Calcium-Carbide-hazards-and-safety
[45] https://edu.rsc.org/download?ac=515424
[46] https://www.nj.gov/health/eoh/rtkweb/documents/fs/0312.pdf
[47] https://www.xiyegroup.com/calcium-carbide-electric-arc-furnace/
[48] https://www.youtube.com/watch?v=H5f15-L1ktA
[49] https://formfunctionart.com/product/calcium-carbide-manufacturing-plant/
[50] https://www.donau-chemie.com/Products-Solutions/BU-Chemie/Kalziumkarbid?lang=en-US
[51] https://www.slideshare.net/slideshow/manufacturing-of-calcium-carbidepdf/262369751
[52] https://nj.gov/health/eoh/rtkweb/documents/fs/0312.pdf
[53] https://chemicalsafety.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=0406&p_version=2
[54] https://www.vedantu.com/question-answer/prepare-acetylene-from-calcium-carbide-class-11-chemistry-cbse-5f853c444dddb9022398bb07
















