Content Menu
● Introduction
● The Chemistry of Tungsten Carbide
>> Elemental Building Blocks
>>> Weight Distribution
>> Hexagonal α-WC
>>> Key Structural Metrics
>> Cubic β-WC
>> Grain Size and Performance
● The Role of Binders: Cemented Carbide
>> Why Binders Matter
>>> Binder Comparison
>> Graded Structures
● Manufacturing Process
>> Step 1: Powder Production
>> Step 2: Mixing and Milling
>> Step 3: Pressing
>> Step 4: Sintering
>> Post-Processing
● Physical and Mechanical Properties
>> Hardness vs. Toughness Trade-Off
>> Thermal Stability
>> Electrical Conductivity
● Applications Across Industries
>> Industrial Cutting Tools
>> Mining and Construction
>> Aerospace
>> Medical
>> Consumer Goods
● Future Trends and Innovations
>> Additive Manufacturing
>> Sustainable Practices
>> Nano-Structured Carbides
● Conclusion
● Frequently Asked Questions (FAQ)
>> 1. Why is tungsten carbide harder than steel?
>> 2. Can tungsten carbide rust?
>> 3. How is tungsten carbide recycled?
>> 4. What's the difference between WC and diamond?
>> 5. Is tungsten carbide toxic?
● Citations:
Introduction
Tungsten carbide is a binary compound of tungsten (W) and carbon (C), but its real-world utility lies in its composite form: cemented carbide. This material dominates industries requiring extreme durability, from aerospace to jewelry. By analyzing its composition, we uncover why it outperforms steel, ceramics, and even diamonds in specific applications.

The Chemistry of Tungsten Carbide
Elemental Building Blocks
Tungsten carbide's formula, WC, reflects a 1:1 atomic ratio. However, the weight distribution skews heavily toward tungsten due to its high atomic mass (183.84 g/mol vs. 12.01 g/mol for carbon).
Weight Distribution
- Tungsten (W): 93–94%
- Carbon (C): 6–6.1%
- Trace Elements: Iron
Hexagonal α-WC
The stable form at room temperature features a hexagonal lattice where each tungsten atom is surrounded by six carbon atoms in a trigonal prism.
Key Structural Metrics
- W–C Bond Length: 220 pm
- Layer Spacing: 284 pm between tungsten layers.
Cubic β-WC
A metastable cubic phase forms above 2530°C but rapidly transitions back to hexagonal upon cooling.
Grain Size and Performance
- Fine grains (0.5–1 µm): Higher hardness, suitable for precision tools.
- Coarse grains (5–10 µm): Better fracture toughness, ideal for mining tools.
The Role of Binders: Cemented Carbide
Why Binders Matter
Pure WC is brittle. Adding 5–25% binder metals (e.g., cobalt, nickel) creates a ductile matrix that holds WC grains together, enabling machining and impact resistance.
Binder Comparison
| Binder | Advantages | Limitations |
| Co | High wettability, cost-effective | Poor corrosion resistance |
| Ni | Corrosion-resistant | Lower toughness than Co |
| Ni-Cr | Enhanced oxidation resistance | Higher cost |
Graded Structures
Advanced cemented carbides use functionally graded designs:
- Surface layer: High WC (94%) for wear resistance.
- Core: Higher binder (15–20%) for shock absorption.
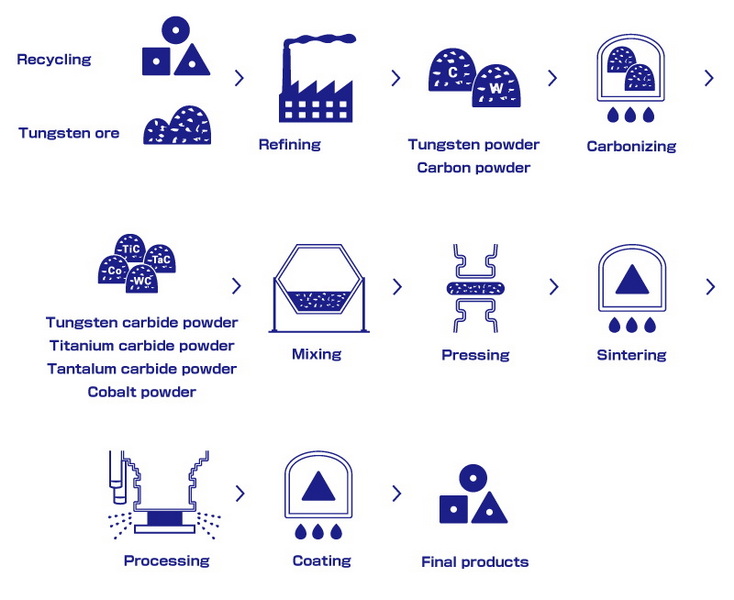
Manufacturing Process
Step 1: Powder Production
- Raw Materials: Tungsten oxide (WO₃) or ammonium paratungstate (APT).
- Reduction: Hydrogen reduces WO₃ to tungsten powder at 700–1000°C.
- Carburization: Carbon black reacts with tungsten at 1400–1600°C.
Step 2: Mixing and Milling
WC powder is ball-milled with binder metals and organic additives (paraffin, PEG) to ensure homogeneity.
Step 3: Pressing
- Uniaxial pressing: For simple shapes (e.g., inserts).
- Cold Isostatic Pressing (CIP): For complex geometries.
Step 4: Sintering
- Vacuum sintering: Prevents oxidation at 1400–1600°C.
- Hot Isostatic Pressing (HIP): Enhances density and eliminates porosity.
Post-Processing
- Grinding: Diamond wheels achieve micron-level precision.
- Coating: Physical vapor deposition (PVD) adds TiN or Al₂O₃ layers for enhanced performance.
Physical and Mechanical Properties
Hardness vs. Toughness Trade-Off
- Hardness: Ranges from 1300 HV (high binder) to 2600 HV (low binder).
- Fracture Toughness: 8–15 MPa·m⊃1;/⊃2;, depending on binder content.
Thermal Stability
- Oxidation Resistance: Stable up to 500°C in air; degrades to WO₃ above 600°C.
- Thermal Conductivity: 110 W/m·K (superior to steel), enabling heat dissipation in cutting tools.
Electrical Conductivity
WC is electrically conductive (resistivity ~0.2 µΩ·m), allowing electro-discharge machining (EDM).
Applications Across Industries
Industrial Cutting Tools
- Inserts: Coated WC-Co grades dominate turning, milling, and drilling.
- Circular Saws: WC-tipped teeth cut composites and metals.
Mining and Construction
- Drill Bits: Coarse-grained WC withstands rock abrasion.
- Tunnel Boring Machines: WC teeth on cutter heads.
Aerospace
- Turbine Blades: WC coatings protect against erosion.
- Rocket Nozzles: High-temperature stability.
Medical
- Surgical Tools: WC-Cr₃C₂ coatings reduce bacterial adhesion.
- Dental Burs: Precision grinding of enamel.
Consumer Goods
- Jewelry: Hypoallergenic WC rings (Ni-binder-free).
- Sports Equipment: Golf club inserts for durability.
Future Trends and Innovations
Additive Manufacturing
- Binder Jetting: 3D-printed WC parts with complex geometries.
- Laser Powder Bed Fusion: High-density WC-Ni components for aerospace.
Sustainable Practices
- Recycling: Recovering cobalt and tungsten from scrap carbide.
- Alternative Binders: Iron-nickel alloys to reduce cobalt dependency.
Nano-Structured Carbides
- Nano-WC (50–100 nm): 20% higher hardness than conventional grades.
- Graphene-Reinforced WC: Enhanced fracture toughness.
Conclusion
Tungsten carbide's composition—94% tungsten and 6% carbon—belies its complexity. Through strategic alloying with binders like cobalt, it achieves a balance of hardness and toughness unmatched by most materials. Innovations in additive manufacturing and nano-engineering promise to expand its applications further, solidifying its role as a linchpin of modern industry.

Frequently Asked Questions (FAQ)
1. Why is tungsten carbide harder than steel?
WC's covalent W–C bonds are stronger than metallic bonds in steel. Its hexagonal lattice also resists dislocation movement.
2. Can tungsten carbide rust?
Pure WC is corrosion-resistant, but cobalt-bonded grades may oxidize in acidic environments. Nickel binders improve corrosion resistance.
3. How is tungsten carbide recycled?
Scrap carbide is crushed, oxidized to WO₃, and reduced back to tungsten. Cobalt is recovered via hydrometallurgy.
4. What's the difference between WC and diamond?
Diamond (10 Mohs) is harder than WC (9–9.5 Mohs), but WC outperforms diamond in high-temperature applications (>600°C).
5. Is tungsten carbide toxic?
WC dust is hazardous if inhaled, but sintered parts are biologically inert. Nickel-bonded grades avoid cobalt-related toxicity concerns.
Citations:
[1] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[2] https://www.insaco.com/material/tungsten-carbide/
[3] https://www.vedantu.com/chemistry/tungsten-carbide
[4] https://scienceinfo.com/tungsten-carbide-properties-applications/
[5] https://www.totalmateria.com/en-us/articles/tungsten-carbide-metals-1/
[6] https://www.mdpi.com/2075-4701/11/12/2035
[7] https://shop.machinemfg.com/the-pros-and-cons-of-tungsten-carbide-a-comprehensive-guide/
[8] https://www.refractorymetal.org/tungsten-carbide-uses-properties.html
[9] https://collegedunia.com/exams/tungsten-carbide-synthesis-properties-and-toxicity-chemistry-articleid-5537
[10] https://en.wikipedia.org/wiki/Tungsten_carbide
[11] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[12] https://www.chemicalbook.com/article/crystal-structure-and-uses-of-tungsten-carbide.htm
[13] https://en.wikipedia.org/wiki/File:-Alpha_tungsten_carbide_crystal_structure.jpg
[14] https://www.diva-portal.org/smash/get/diva2:1237216/FULLTEXT01.pdf
[15] https://www.retopz.com/57-frequently-asked-questions-faqs-about-tungsten-carbide/
[16] https://hpvchemicals.oecd.org/ui/handler.axd?id=ed1c76bf-dad9-4baa-8d1b-70fed7f92862
[17] https://www.star-su.com/wp-content/uploads/HB-Carbide-Grade-Chart-and-Data-Sheets.pdf
[18] http://hardmetal-engineering.blogspot.com/2011/
[19] http://www.carbidetechnologies.com/faq/what-is-tungsten-carbide/
[20] https://carbosystem.com/en/tungsten-carbide/
[21] https://www.hitechseals.com/includes/pdf/tungsten_carbide.pdf
[22] https://pubchem.ncbi.nlm.nih.gov/compound/Tungsten-carbide
[23] https://www.harcourt.co/overview_documents/Tungsten%20Carbide%20data%20sheet.PDF
[24] https://www.woksal.com/dokumenta/WOKSAL-HARD-METAL.pdf
[25] https://www.dymetalloys.co.uk/what-is-tungsten-carbide
[26] https://www.refractorymetal.org/physical-chemical-properties-of-tungsten.html
[27] https://next-gen.materialsproject.org/materials/mp-1894
[28] https://pubchem.ncbi.nlm.nih.gov/compound/tungsten_carbide
[29] https://www.imetra.com/tungsten-carbide-material-properties/
[30] https://pubchem.ncbi.nlm.nih.gov/compound/tungsten-carbide-_W2C
[31] https://rrcarbide.com/understanding-tungsten-carbide-composition-uses-and-expertise/
[32] https://www.carbideprobes.com/wp-content/uploads/2019/07/TungstenCarbideDataSheet.pdf
[33] https://www.dymetalloys.co.uk/what-is-tungsten-carbide/grade-chart
[34] http://www.machiningtech.com/tungsten-carbide-grade-Chart.html
[35] https://www.hyperionmt.com/en/products/Carbide-Rolls/grade-data/
[36] https://www.istockphoto.com/photo/crystaline-structure-of-tungsten-carbide-gm166044155-17867637
[37] https://www.generalcarbide.com/wp-content/uploads/2019/04/GeneralCarbide-Designers_Guide_TungstenCarbide.pdf
[38] https://commons.wikimedia.org/wiki/File:-Alpha_tungsten_carbide_crystal_structure.jpg
[39] https://www.sciencedirect.com/science/article/pii/S0263436823002019
[40] https://cen.acs.org/materials/Chemistry-Pictures-Tungsten-carbide-slice/103/web/2025/02
[41] https://www.sciencedirect.com/science/article/pii/S026343681830533X
[42] https://www.atomic-scale-physics.de/lattice/struk/bh.html
[43] https://www.tungco.com/insights/blog/frequently-asked-questions-used-tungsten-carbide-inserts/
[44] https://www.tungstenworld.com/pages/tungsten-news-common-questions-about-tungsten
[45] https://en.wikipedia.org/wiki/Tungsten_carbide
[46] https://tuncomfg.com/about/faq/
[47] https://www.zzbetter.com/new/Understanding-the-Composition-and-Properties-of-Tungsten-Carbide-and-Titanium-Carbide.html
[48] http://www.carbidetechnologies.com/faqs/
[49] https://www.hit-tw.com/newsdetails.aspx?nid=298
[50] https://shop.machinemfg.com/tungsten-carbide-an-overview/
[51] https://www.vedantu.com/chemistry/tungsten-carbide
[52] https://www.linkedin.com/pulse/questions-composite-materials-tungsten-carbide-shijin-lei
[53] https://www.aemmetal.com/news/tungsten-vs-tungsten-carbide-guide.html
[54] https://www.insaco.com/material/tungsten-carbide/
















