Content Menu
● Introduction to Tungsten Carbide
● Raw Materials Used in Tungsten Carbide Production
>> Tungsten Ore
>> Ammonium Paratungstate (APT)
>> Tungsten Oxide
>> Tungsten Metal Powder
>> Carbon Sources
>> Binders (Cobalt, Nickel, Iron)
>> Additives and Forming Agents
● The Chemistry Behind Tungsten Carbide Formation
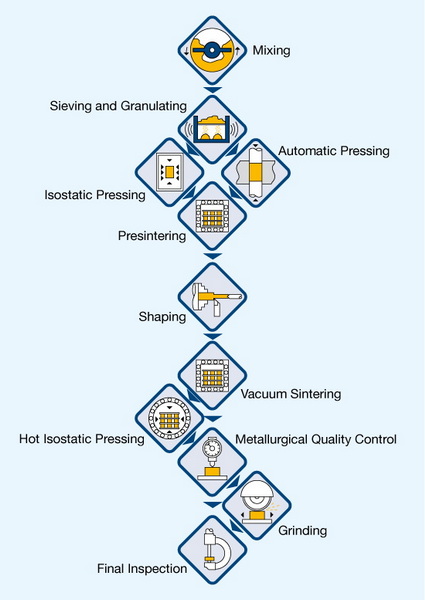
● Manufacturing Process: Step-by-Step
>> 1. Ore Refinement and Preparation
>> 2. Reduction to Tungsten Powder
>> 3. Mixing with Carbon
>> 4. Carburization
>> 5. Milling and Sieving
>> 6. Addition of Binders
>> 7. Shaping and Compaction
>> 8. Sintering
>> 9. Post-Processing
● Role of Binders and Additives
● Quality Control and Grain Size Management
● Applications of Tungsten Carbide
● Environmental and Recycling Considerations
● Advances in Tungsten Carbide Technology
● Challenges in Tungsten Carbide Production
● Future Prospects
● Conclusion
● FAQ: Related Questions About Tungsten Carbide
>> 1. What is the chemical formula of tungsten carbide?
>> 2. Why is cobalt used as a binder in tungsten carbide production?
>> 3. Can tungsten carbide be recycled?
>> 4. What determines the hardness of tungsten carbide?
>> 5. What are the main industrial uses of tungsten carbide?
Tungsten carbide stands as one of the hardest and most durable materials in modern industry. Its unique blend of extreme hardness, high density, and resistance to wear makes it indispensable for a broad range of applications, from cutting tools and abrasives to jewelry and armor-piercing ammunition. But what exactly is used to make tungsten carbide, and how does raw ore transform into this remarkable compound? In this comprehensive guide, we will explore the raw materials, chemistry, and manufacturing processes behind tungsten carbide, illustrated with detailed descriptions and visual explanations.

Introduction to Tungsten Carbide
Tungsten carbide (WC) is a compound consisting of equal parts tungsten and carbon atoms. In its purest form, it appears as a fine, gray powder. Through advanced manufacturing techniques, this powder is transformed into solid forms that are exceptionally hard and wear-resistant, rivaling even diamond in certain properties.
Key Properties:
- Hardness: Comparable to diamond
- Density: About twice that of steel
- Melting Point: Approximately 2,780°C
- Wear Resistance: Extremely high
Tungsten carbide's unique characteristics make it a material of choice for demanding industrial applications.
Raw Materials Used in Tungsten Carbide Production
The journey of tungsten carbide begins with a careful selection of raw materials. Each ingredient plays a critical role in the final product's quality and performance.
Tungsten Ore
The primary source of tungsten is tungsten ore, which is mined from the earth. The most common ores include scheelite (CaWO₄) and wolframite ((Fe,Mn)WO₄). These ores are processed to extract tungsten trioxide (WO₃), a crucial precursor for tungsten carbide production.
Ammonium Paratungstate (APT)
APT is a purified, crystalline compound derived from tungsten ore. It acts as an intermediate in the production of tungsten metal and ultimately tungsten carbide. APT is converted into tungsten oxide through calcination at high temperatures.
Tungsten Oxide
Tungsten oxide (WO₃) is produced by heating APT. This yellow or blue powder is then reduced to metallic tungsten powder in a hydrogen atmosphere.
Tungsten Metal Powder
The reduction of tungsten oxide yields pure tungsten metal powder, which serves as the primary tungsten source for carbide production.
Carbon Sources
Carbon is the second essential element in tungsten carbide. Common sources include:
- Carbon black (soot)
- Graphite powder
The purity and particle size of the carbon source are critical to ensure a complete and uniform reaction with tungsten.
Binders (Cobalt, Nickel, Iron)
While pure tungsten carbide is extremely hard, it is also brittle. To enhance toughness and workability, powdered binders such as cobalt, nickel, or iron are added. Cobalt is the most widely used binder, providing excellent wetting and binding properties during sintering.
Additives and Forming Agents
Additional materials may be used during processing, including:
- Forming agents (e.g., wax) to aid in shaping
- Liquids (water, ethanol) for wet milling
- Inert gases (argon, nitrogen) to prevent unwanted reactions
The Chemistry Behind Tungsten Carbide Formation
The fundamental chemical reaction that forms tungsten carbide is a high-temperature combination of tungsten and carbon:
W+C→WC
This reaction typically occurs at temperatures between 1,400°C and 2,000°C. The precise control of temperature and reactant ratios is essential to ensure the formation of stoichiometric WC with minimal impurities.
Manufacturing Process: Step-by-Step
The production of tungsten carbide is a sophisticated, multi-stage process that combines chemistry, material science, and precision engineering.
1. Ore Refinement and Preparation
- Mining: Tungsten ore is extracted from open-pit or underground mines.
- Concentration: The ore is crushed and concentrated to increase tungsten content.
- Chemical Processing: The concentrated ore is converted into ammonium paratungstate (APT), then calcined to produce tungsten oxide.
2. Reduction to Tungsten Powder
- Reduction Furnace: Tungsten oxide is heated in a hydrogen atmosphere, reducing it to pure tungsten powder.
- Quality Control: The particle size and purity of the tungsten powder are carefully monitored.
3. Mixing with Carbon
- Batch Calculation: The exact amount of carbon needed is calculated, accounting for any oxygen present in the tungsten powder.
- Ball Milling: Tungsten powder and carbon black are mixed in a ball mill for several hours to ensure uniform distribution.
4. Carburization
- High-Temperature Furnace: The mixture is heated in a graphite or vacuum furnace at 1,300–1,600°C.
- Reaction: Tungsten reacts with carbon to form tungsten carbide powder.
5. Milling and Sieving
- Ball Milling: The resulting carbide powder is milled to achieve the desired particle size.
- Sieving: The powder is sieved to separate fine, medium, and coarse particles.
6. Addition of Binders
- Binder Mixing: Cobalt (or another binder) powder is mixed with the tungsten carbide powder.
- Wet Milling: Water or ethanol may be added to improve mixing and prevent oxidation.
7. Shaping and Compaction
- Forming Agents: Wax or other agents are added to aid in pressing.
- Pressing: The mixture is pressed into molds, forming a “green body” with the desired shape.
8. Sintering
- Vacuum or Inert Atmosphere: The green bodies are sintered in a furnace at 1,350–1,500°C.
- Binder Melting: The binder melts, wets, and binds the tungsten carbide particles together.
- Shrinkage: The component shrinks by up to 25% as it densifies.
9. Post-Processing
- Machining: The sintered parts are machined to final dimensions using diamond or cubic boron nitride tools.
- Quality Inspection: Finished products undergo rigorous testing for hardness, density, and microstructure.
Role of Binders and Additives
The addition of a binder metal, usually cobalt, is crucial for transforming brittle tungsten carbide powder into tough, workable components. During sintering, the binder melts and flows around the carbide grains, creating a strong, cohesive structure with enhanced toughness and resistance to fracture.
Other additives, such as forming agents and inert gases, facilitate shaping, pressing, and sintering while minimizing oxidation and contamination.
Quality Control and Grain Size Management
Grain size plays a pivotal role in determining the mechanical properties of tungsten carbide. Fine-grained carbides offer higher hardness and wear resistance, while coarser grains provide greater toughness. Manufacturers carefully control the particle size of both tungsten and carbide powders, as well as the sintering conditions, to tailor properties for specific applications.
Applications of Tungsten Carbide
Tungsten carbide's unique combination of hardness, toughness, and wear resistance makes it invaluable in numerous fields:
- Cutting Tools: Drills, end mills, lathe inserts, and saw blades
- Mining and Drilling Equipment: Wear-resistant tips and bits
- Industrial Machinery: Bearings, nozzles, and dies
- Abrasives: Grinding wheels and powders
- Defense: Armor-piercing projectiles
- Jewelry: Rings and watch components
Its ability to maintain sharp edges and withstand extreme conditions has revolutionized manufacturing, construction, and even consumer goods.
Environmental and Recycling Considerations
Given the strategic importance and cost of tungsten, recycling plays a significant role in the tungsten carbide industry. Scrap carbide tools and components are collected, processed, and regenerated into high-quality tungsten carbide powder, reducing reliance on primary raw materials and minimizing environmental impact.
Recycling not only conserves valuable resources but also reduces the environmental footprint associated with mining and refining tungsten ores. Modern recycling methods can recover both tungsten and binder metals, ensuring that the reclaimed materials perform as well as those made from virgin sources.
Advances in Tungsten Carbide Technology
Recent advancements in tungsten carbide technology have focused on improving its performance and expanding its applications. Innovations in nano-grain tungsten carbide powders have led to materials with even greater hardness and toughness, enabling tools to last longer and perform better under extreme conditions.
Researchers are also exploring new binder materials and composite structures to enhance thermal stability and resistance to oxidation, which are critical for high-speed machining and harsh environments.
The development of coated tungsten carbide tools, where the carbide core is covered with ultra-hard ceramics like titanium nitride or aluminum oxide, has further improved tool life and cutting performance. These coatings reduce friction, resist heat, and protect the underlying carbide from chemical attack.
Challenges in Tungsten Carbide Production
Despite its many advantages, producing tungsten carbide presents several challenges. The high temperatures required for sintering demand significant energy consumption, and controlling the grain size and purity requires precise manufacturing conditions.
Additionally, the brittleness of tungsten carbide composites can lead to cracking under impact or stress, necessitating ongoing research into improving toughness without sacrificing hardness.
Another challenge lies in the cost and availability of raw materials. Tungsten is a strategic metal with limited global sources, making supply chain stability and recycling increasingly important for manufacturers.
Future Prospects
The future of tungsten carbide looks promising, with ongoing research aimed at developing more sustainable production methods and recycling techniques. The integration of tungsten carbide with other advanced materials, such as ceramics and superalloys, is expected to open new frontiers in aerospace, automotive, and medical device industries.
Emerging applications, such as additive manufacturing (3D printing) of tungsten carbide components, are being explored to create complex shapes with minimal waste. As technology advances, tungsten carbide will continue to play a vital role in pushing the boundaries of what is possible in material science and engineering.
Conclusion
Tungsten carbide is a marvel of modern materials science, created through the precise combination of tungsten and carbon, along with carefully selected binders and additives. From the mining of tungsten ore to the final sintering of shaped components, every step in the process is meticulously engineered to produce a material that is both incredibly hard and remarkably tough. As industries continue to push the boundaries of performance and durability, tungsten carbide remains at the forefront, enabling innovation across countless applications.

FAQ: Related Questions About Tungsten Carbide
1. What is the chemical formula of tungsten carbide?
The chemical formula of tungsten carbide is WC, indicating it contains equal parts tungsten and carbon atoms.
2. Why is cobalt used as a binder in tungsten carbide production?
Cobalt is used as a binder because it melts at a lower temperature than tungsten carbide, wets the carbide grains during sintering, and imparts toughness to the otherwise brittle material.
3. Can tungsten carbide be recycled?
Yes, tungsten carbide is highly recyclable. Scrap carbide tools and components are processed to recover tungsten and regenerate high-quality carbide powder for new products.
4. What determines the hardness of tungsten carbide?
The hardness of tungsten carbide is determined by its grain size, purity, and the ratio of tungsten to carbon. Fine-grained carbides are generally harder and more wear-resistant.
5. What are the main industrial uses of tungsten carbide?
Tungsten carbide is widely used in cutting tools, mining and drilling equipment, industrial machinery, abrasives, defense applications, and jewelry due to its exceptional hardness and wear resistance.
















