Content Menu
● Introduction to Tungsten Carbide
● Chemical Composition of Tungsten Carbide
>> Elemental Breakdown
● The Manufacturing Process
>> 1. Extraction and Preparation of Tungsten
>> 2. Carburization
>> 3. Powder Processing
>> 4. Compaction and Sintering
>> 5. Finishing
● Structural Properties and Forms
● Key Physical and Chemical Properties
● Industrial Applications
>> 1. Cutting and Machining Tools
>> 2. Mining and Construction
>> 3. Aerospace and Defense
>> 4. Oil and Gas
>> 5. Medical Technology
>> 6. Jewelry
● Environmental and Recycling Considerations
● Future Trends in Tungsten Carbide Technology
● Conclusion
● FAQ: Common Questions About Tungsten Carbide
>> 1. What is tungsten carbide made out of?
>> 2. How is tungsten carbide different from steel?
>> 3. What are the main uses of tungsten carbide?
>> 4. Can tungsten carbide be recycled?
>> 5. Is tungsten carbide safe to handle and use?
Tungsten carbide is one of the most remarkable materials in modern engineering, known for its exceptional hardness, durability, and versatility. But what exactly is tungsten carbide made out of? In this comprehensive article, we will explore the chemical composition, manufacturing process, structural properties, and diverse applications of tungsten carbide. Along the way, we will include visual representations to help illustrate these concepts, provide a detailed FAQ section, and conclude with a summary of key points.

Introduction to Tungsten Carbide
Tungsten carbide is a compound that has revolutionized industries ranging from manufacturing and mining to jewelry and aerospace. Its unique combination of hardness, strength, and resistance to wear makes it indispensable in applications where ordinary metals would fail. First synthesized in the late 19th century, tungsten carbide gained prominence during World War II as a strategic material for armor-piercing ammunition. Today, it is a cornerstone of modern engineering, enabling advancements in precision machining, renewable energy, and even medical technology.
Chemical Composition of Tungsten Carbide
At its core, tungsten carbide is a chemical compound made from two elements: tungsten (W) and carbon (C). The most common form has a 1:1 atomic ratio, resulting in the chemical formula WC. This means each molecule of tungsten carbide contains one tungsten atom and one carbon atom, tightly bonded together to form a dense, hard material.
Elemental Breakdown
- Tungsten (W): A heavy, hard, and dense metal known for its high melting point (3,422°C) and strength. Tungsten is extracted from minerals like wolframite and scheelite.
- Carbon (C): A nonmetal element that, when bonded with tungsten, imparts significant hardness and wear resistance.
In industrial applications, tungsten carbide is rarely used in its pure form. Instead, it is typically combined with a metallic binder-most commonly cobalt (Co), but sometimes nickel (Ni) or iron (Fe)-to create a composite material known as cemented carbide. The binder holds the tungsten carbide grains together, enhancing toughness and making the material easier to shape and use in tools or components.
Typical Composition in Cemented Carbide:
| Component | Percentage by Weight |
| Tungsten Carbide | 80–97% |
| Cobalt (Binder) | 3–20% |
| Nickel/Iron | Trace (optional) |
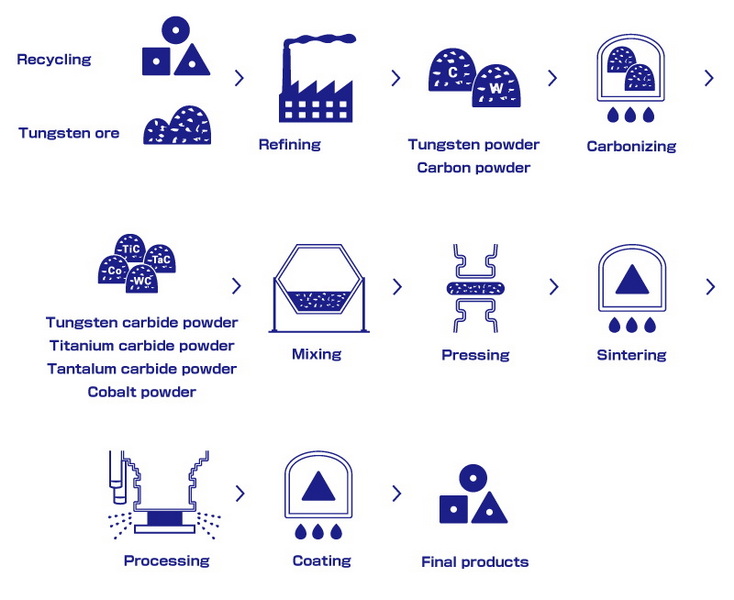
The Manufacturing Process
The production of tungsten carbide is a sophisticated process that combines advanced chemistry with precision engineering. Here's a step-by-step overview of how tungsten carbide is made:
1. Extraction and Preparation of Tungsten
- Tungsten Ore: The process begins with the extraction of tungsten ore, such as scheelite (CaWO₄) or wolframite ((Fe,Mn)WO₄), from the earth.
- Ammonium Paratungstate (APT): The ore is crushed, treated with chemicals, and refined into ammonium paratungstate, a purified intermediate.
- Tungsten Oxide: APT is calcined at high temperatures (500–1,000°C) to produce tungsten oxide (WO₃).
- Tungsten Metal Powder: Tungsten oxide is reduced in a hydrogen atmosphere at 700–1,200°C to obtain pure tungsten metal powder.
2. Carburization
- Mixing with Carbon: The tungsten powder is thoroughly mixed with a carbon source, such as graphite or soot, in precise ratios.
- High-Temperature Reaction: This mixture is heated in a furnace at temperatures between 1,400°C and 2,000°C. The tungsten reacts with carbon to form tungsten carbide (WC) powder.
3. Powder Processing
- Milling: The resulting tungsten carbide powder is milled to achieve the desired particle size (typically 0.5–10 micrometers) and uniformity.
- Blending with Binder: The powder is mixed with a metallic binder (usually cobalt) to enhance toughness and facilitate sintering.
4. Compaction and Sintering
- Pressing: The blended powder is pressed into the desired shapes using hydraulic or mechanical molds.
- Sintering: The pressed shapes are heated in a sintering furnace at 1,400°C–1,600°C. The binder melts and flows around the tungsten carbide grains, cementing them together into a dense, solid mass.
5. Finishing
- Machining and Grinding: The sintered parts are machined and ground to precise dimensions and surface finishes, often using diamond tools due to the material's extreme hardness.
- Coatings (Optional): Some components receive additional coatings, such as titanium nitride (TiN), to further enhance wear resistance.
Structural Properties and Forms
Tungsten carbide exists in two primary crystalline forms:
- Hexagonal (α-WC): The most common and stable form at room temperature, featuring layers of tungsten atoms with carbon atoms occupying half the interstitial sites.
- Cubic (β-WC): A high-temperature form with a rock-salt structure, less commonly encountered in industrial applications.
The strong covalent bonds between tungsten and carbon atoms give the material exceptional hardness, comparable to that of diamond, and a high degree of stiffness and density.
Key Physical and Chemical Properties
Tungsten carbide's unique properties stem from its composition and structure:
- Hardness: Ranks 9–9.5 on the Mohs scale, just below diamond.
- Density: Approximately 15.6 g/cm³, much denser than steel (7.8 g/cm³).
- Melting Point: Around 2,870°C, allowing it to withstand extreme temperatures.
- Young's Modulus: 530–700 GPa, indicating high stiffness (three times that of steel).
- Thermal Expansion: Low coefficient of thermal expansion (5.5 µm/m·K), reducing deformation under heat.
- Wear Resistance: Exceptional resistance to abrasion and deformation, even under high-stress conditions.
- Corrosion Resistance: Stable in most environments, though susceptible to attack by strong acids like hydrofluoric acid.
- Thermal Conductivity: Efficient at dissipating heat (110 W/m·K), ideal for high-speed machining.
Industrial Applications
Tungsten carbide's extraordinary properties make it invaluable in a wide range of industries:
1. Cutting and Machining Tools
- Drills, Milling Inserts, and Lathe Tools: Tungsten carbide's hardness allows for precise, high-speed machining of metals, plastics, and composites.
- Circular Saw Blades: Used in woodworking and metalworking for clean, durable cuts.
2. Mining and Construction
- Drill Bits and Excavation Tools: Essential for drilling through hard rock in mining and tunneling.
- Wear Parts: Used in crushers, conveyor systems, and hydraulic components to resist abrasion.
3. Aerospace and Defense
- Armor-Piercing Ammunition: Tungsten carbide cores penetrate armored targets effectively.
- Turbine Components: Withstands high temperatures and stresses in jet engines.
4. Oil and Gas
- Valve Seats and Nozzles: Resists erosion in high-pressure drilling environments.
5. Medical Technology
- Surgical Tools: Scalpels and dental drills benefit from carbide's sharpness and sterilizability.
- Prosthetics: Used in joint replacements due to biocompatibility and wear resistance.
6. Jewelry
- Rings and Watches: Polished to a permanent luster, resistant to scratches and tarnishing.
Environmental and Recycling Considerations
Tungsten carbide is highly recyclable, with up to 95% of scrap material recoverable. Recycling processes involve:
1. Collection: Scrap tools and manufacturing waste are gathered.
2. Chemical Treatment: Binder metals are dissolved, separating tungsten carbide powder.
3. Reuse: The purified powder is reintroduced into the manufacturing cycle.
This circular approach reduces reliance on mining and lowers the carbon footprint of carbide production.
Future Trends in Tungsten Carbide Technology
1. Nanostructured Carbides: Nanoparticles of WC are being researched for coatings and composites, offering enhanced strength and flexibility.
2. Binder Innovations: Development of eco-friendly binders, such as iron-nickel alloys, to replace cobalt in certain applications.
3. Additive Manufacturing: 3D printing of complex tungsten carbide components using binder-jet or laser sintering techniques.
Conclusion
Tungsten carbide is a fascinating material, engineered from the fusion of tungsten and carbon atoms in a precise ratio. Its unique combination of extreme hardness, density, and resistance to wear and heat has made it a cornerstone of modern industry. From cutting tools and mining equipment to jewelry and aerospace components, tungsten carbide's versatility and performance are unmatched. As technology advances, innovations in nanostructured carbides, sustainable binders, and additive manufacturing promise to expand its applications further. Understanding its composition and production not only highlights the ingenuity of material science but also underscores the critical role tungsten carbide plays in advancing technology and industry.

FAQ: Common Questions About Tungsten Carbide
1. What is tungsten carbide made out of?
Tungsten carbide is made from a chemical combination of tungsten and carbon atoms in a 1:1 ratio. In most industrial applications, a metallic binder such as cobalt or nickel is added to improve toughness and facilitate manufacturing.
2. How is tungsten carbide different from steel?
Tungsten carbide is significantly harder and denser than steel. It maintains its sharpness and resists wear much better, making it ideal for cutting tools and high-stress applications where steel would quickly degrade.
3. What are the main uses of tungsten carbide?
Tungsten carbide is widely used in cutting and drilling tools, mining equipment, wear-resistant parts, aerospace components, medical instruments, and jewelry.
4. Can tungsten carbide be recycled?
Yes, tungsten carbide is highly recyclable. Scrap materials are processed to recover tungsten and binder metals, reducing environmental impact.
5. Is tungsten carbide safe to handle and use?
Tungsten carbide is generally safe in finished products. However, inhalation of fine powders during manufacturing requires protective measures.
















