Content Menu
● Introduction to Tungsten Carbide
>> Key Properties of Tungsten Carbide
● What Defines Non-Ferrous Metals?
● Tungsten Carbide: Metal, Ceramic, or Composite?
>> 1. Chemical Composition
>> 2. Why It's Not a Non-Ferrous Metal
● Manufacturing Process of Tungsten Carbide
>> Step 1: Raw Material Preparation
>> Step 2: Mixing with Binder
>> Step 3: Compaction
>> Step 4: Sintering
● Industrial and Commercial Applications
>> 1. Machining and Cutting Tools
>> 2. Aerospace and Defense
>> 3. Medical Equipment
>> 4. Consumer Goods
>> 5. Energy Sector
● Tungsten Carbide vs. Competing Materials
● Environmental Impact and Recycling
● Global Market Insights
● Future Innovations
● Conclusion
● Frequently Asked Questions
>> 1. What is the difference between tungsten and tungsten carbide?
>> 2. Can tungsten carbide rust or tarnish?
>> 3. How does tungsten carbide compare to diamond in hardness?
>> 4. Is tungsten carbide toxic?
>> 5. What industries rely most on tungsten carbide?
● Citations:
Tungsten carbide (WC) has revolutionized industries with its unparalleled hardness and durability. While its applications span from industrial machinery to luxury jewelry, a fundamental question persists: Is tungsten carbide a non-ferrous metal? This article explores its composition, classification, and industrial significance while debunking common misconceptions.

Introduction to Tungsten Carbide
Tungsten carbide is a compound composed of tungsten (W) and carbon (C) atoms in a 1:1 ratio. First synthesized in the late 19th century, it gained prominence during World War II for its use in armor-piercing ammunition. Today, it is a cornerstone material in high-performance engineering.
Key Properties of Tungsten Carbide
- Hardness: Ranks 9–9.5 on the Mohs scale, surpassed only by diamond.
- Density: ~15.6 g/cm³, nearly double that of steel.
- Melting Point: 2,870°C, ideal for extreme environments.
- Corrosion Resistance: Resists oxidation and chemical degradation, except in hydrofluoric acid.
What Defines Non-Ferrous Metals?
Non-ferrous metals are alloys or pure metals lacking iron as a primary component. Examples include aluminum, copper, and zinc. They are prized for their:
- Lightweight: Critical for aerospace and automotive industries.
- Corrosion Resistance: Suitable for marine and chemical environments.
- Electrical Conductivity: Copper and aluminum dominate wiring and electronics.
Ferrous vs. Non-Ferrous Metals
| Property | Ferrous Metals (e.g., Steel) | Non-Ferrous Metals (e.g., Aluminum) |
| Iron Content | High | None or negligible |
| Corrosion Resistance | Prone to rust | Highly resistant |
| Density | High (7.8 g/cm³ for steel) | Low (2.7 g/cm³ for aluminum) |
Tungsten Carbide: Metal, Ceramic, or Composite?
Tungsten carbide's classification is often debated due to its hybrid nature:
1. Chemical Composition
WC is a ceramic compound, not a pure metal. However, in industrial applications, it is combined with a metallic binder (e.g., cobalt or nickel) to form cemented carbide—a cermet (ceramic-metal composite).
2. Why It's Not a Non-Ferrous Metal
- Lacks Metallic Bonding: Pure WC has covalent bonds, unlike metals' metallic bonds.
- Requires a Binder: Its structural integrity depends on a metal matrix.
- Primary Use as a Ceramic: Dominates applications requiring hardness over malleability.
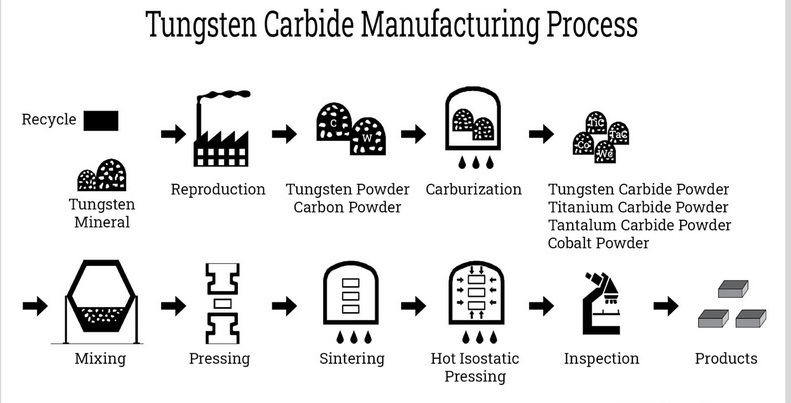
Manufacturing Process of Tungsten Carbide
The production of cemented carbide involves advanced powder metallurgy techniques:
Step 1: Raw Material Preparation
- Tungsten ore (e.g., wolframite) is refined into tungsten powder via hydrogen reduction.
- Carbon black is added to form tungsten carbide powder.
Step 2: Mixing with Binder
- WC powder is blended with 5–20% cobalt/nickel binder to enhance toughness.
Step 3: Compaction
- The mixture is pressed into molds under 100–400 MPa pressure to form “green” parts.
Step 4: Sintering
- Parts are heated at 1,300–1,500°C in a vacuum furnace, causing the binder to melt and bond the WC particles.
Industrial and Commercial Applications
Tungsten carbide's versatility makes it indispensable across sectors:
1. Machining and Cutting Tools
- End Mills and Inserts: WC-coated tools extend lifespan in CNC machining.
- Mining Drill Bits: Outperforms steel in abrasive rock drilling.
2. Aerospace and Defense
- Turbine blade coatings withstand extreme temperatures.
- Armor-piercing projectiles leverage WC's density.
3. Medical Equipment
- Surgical tools (e.g., scalpels) retain sharpness longer than stainless steel.
- Dental drills minimize patient discomfort due to precision.
4. Consumer Goods
- Jewelry: Scratch-resistant wedding bands (Image: Polished tungsten carbide ring).
- Sporting Goods: Golf clubs and fishing weights utilize WC for durability.
5. Energy Sector
- Oil and gas drill bits endure high-pressure environments.
Tungsten Carbide vs. Competing Materials
| Material | Hardness (Mohs) | Density (g/cm³) | Melting Point (°C) |
| Tungsten Carbide | 9–9.5 | 15.6 | 2,870 |
| Diamond | 10 | 3.5 | 3,550 (sublimates) |
| Titanium | 6 | 4.5 | 1,668 |
| High-Speed Steel | 7–8 | 8.0 | 1,370 |
Environmental Impact and Recycling
Tungsten carbide is 100% recyclable. Recycling processes include:
1. Mechanical Reclamation: Crushing and grinding scrap tools.
2. Chemical Recovery: Dissolving binders to extract WC powder.
3. Re-sintering: Reprocessing powder into new components.
Economic Benefit: Recycling reduces production costs by 30–40% compared to virgin material.
Global Market Insights
The tungsten carbide market is projected to grow at 6.2% CAGR from 2023 to 2030, driven by:
- Demand for energy-efficient machining tools.
- Expansion in aerospace and electronics sectors.
- China dominates production, accounting for 80% of global tungsten supply.
Future Innovations
1. Additive Manufacturing: 3D-printed WC parts for complex geometries.
2. Nano-Structured WC: Enhanced hardness for micro-tools.
3. Binderless WC: Eliminating cobalt for biomedical compatibility.
Conclusion
Tungsten carbide's unique blend of ceramic and metallic properties positions it as a critical material in modern engineering. While not a non-ferrous metal, its role in high-stress applications is irreplaceable. Advances in recycling and nanotechnology promise to further elevate its industrial significance.
Frequently Asked Questions
1. What is the difference between tungsten and tungsten carbide?
Tungsten is a pure metal, while tungsten carbide is a compound of tungsten and carbon. The latter is harder and used in applications requiring extreme durability.
2. Can tungsten carbide rust or tarnish?
No. Unlike ferrous metals, tungsten carbide resists oxidation and tarnishing, making it ideal for marine and medical uses.
3. How does tungsten carbide compare to diamond in hardness?
Diamond scores 10 on the Mohs scale, while tungsten carbide ranks 9–9.5. However, WC outperforms diamond in high-temperature stability.
4. Is tungsten carbide toxic?
In solid form, it is inert and safe. However, inhalation of WC dust during machining can cause lung damage, requiring proper safety measures.
5. What industries rely most on tungsten carbide?
Key sectors include manufacturing (60%), mining (20%), aerospace (10%), and healthcare (5%).
Citations:
[1] https://en.wikipedia.org/wiki/Tungsten_carbide
[2] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[3] https://www.britannica.com/science/tungsten-carbide
[4] https://www.istockphoto.com/photos/tungsten-carbide
[5] https://www.carbide-usa.com/top-5-uses-for-tungsten-carbide/
[6] https://www.retopz.com/57-frequently-asked-questions-faqs-about-tungsten-carbide/
[7] https://www.thermalspray.com/questions-tungsten-carbide/
[8] http://www.chinatungsten.com/Nonferrous-Metals.html
[9] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[10] https://www.tungco.com/insights/blog/5-tungsten-carbide-applications/
[11] https://shop.machinemfg.com/the-pros-and-cons-of-tungsten-carbide-a-comprehensive-guide/
[12] https://eurobalt.net/blog/2022/03/28/all-the-applications-of-tungsten-carbide/
[13] https://www.reddit.com/r/askscience/comments/9whr5d/is_tungsten_carbide_an_alloy/
[14] https://www.bangerter.com/en/tungsten-carbide
[15] https://www.totalmateria.com/en-us/articles/tungsten-carbide-metals-1/
[16] https://www.alamy.com/stock-photo/tungsten-carbide.html
[17] https://periodictable.com/Elements/074/pictures.html
[18] http://www.tungsten-carbide.com.cn
[19] https://www.freepik.com/free-photos-vectors/tungsten-carbide
[20] http://www.chinatungsten.com/Tungsten-Carbide/Properties-of-Tungsten-Carbide.html
[21] http://picture.chinatungsten.com/list-18.html
[22] https://www.samaterials.com/content/application-of-tungsten-in-modern-industry.html
[23] https://www.itia.info/applications-markets/
[24] https://www.carbideprobes.com/wp-content/uploads/2019/07/TungstenCarbideDataSheet.pdf
[25] https://www.vedantu.com/chemistry/tungsten-carbide
[26] https://eternaltools.com/blogs/tutorials/tungsten-carbide-an-informative-guide
[27] http://machinetoolrecyclers.com/rita_hayworth.html
















