Content Menu
● Traditional Production Methods
● Advanced Synthesis Techniques
● Crystal Structure and Polymorphs of Silicon Carbide
● Raw Material Preparation and Processing
● Industrial Applications of Silicon Carbide
● Silicon Carbide in Emerging Technologies
● Production Challenges and Innovations
● Environmental Impact and Mitigation
● Environmental Regulations and Sustainability Efforts
● Market Dynamics and Scalability
● Future Trends
● Final Thoughts on Silicon Carbide Production
● Conclusion
● FAQs
>> 1. What raw materials are essential for silicon carbide production?
>> 2. How does the Acheson process differ from the Lely method?
>> 3. Can silicon carbide be recycled during production?
>> 4. What industries benefit most from reaction-bonded SiC?
>> 5. How do sintering additives improve SiC quality?
Silicon carbide (SiC) is a synthetic ceramic material renowned for its exceptional hardness, thermal stability, and chemical resistance. Its production involves advanced synthesis methods tailored to meet industrial demands across sectors like metallurgy, military, oil drilling, and construction. Below, we explore the key processes, innovations, and applications shaping the production of silicon carbide.
Traditional Production Methods
The Acheson Process
The Acheson method, developed in 1893, remains the backbone of commercial silicon carbide manufacturing. This process combines high-purity silica sand and carbon sources like petroleum coke in a graphite-resistance furnace heated to 2,500°C. The intense heat triggers a reaction where silica reduces to silicon vapor, which bonds with carbon to form SiC crystals.
The resulting material varies in purity based on its proximity to the furnace's graphite core. Colorless or pale crystals near the core exhibit the highest purity, while darker crystals farther out contain impurities like nitrogen or aluminum. Despite its energy intensity, the Acheson process dominates due to its scalability and cost-effectiveness in the production of silicon carbide.
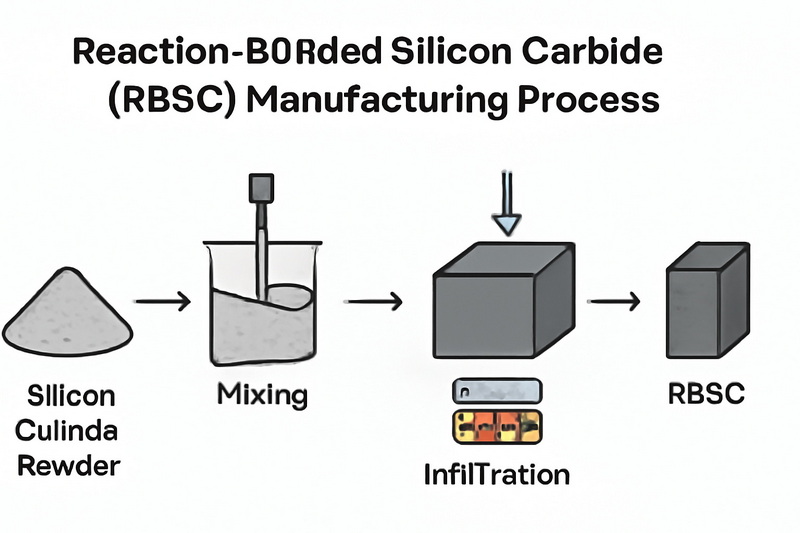
Reaction-Bonded Silicon Carbide (RBSC)
RBSC blends silicon carbide powder with carbon, shaping the mixture into a preform. Liquid silicon infiltrates the preform at high temperatures, reacting with carbon to form additional SiC. This method produces complex, high-strength components with minimal machining requirements, making it ideal for industrial tooling and aerospace parts.
Advanced Synthesis Techniques
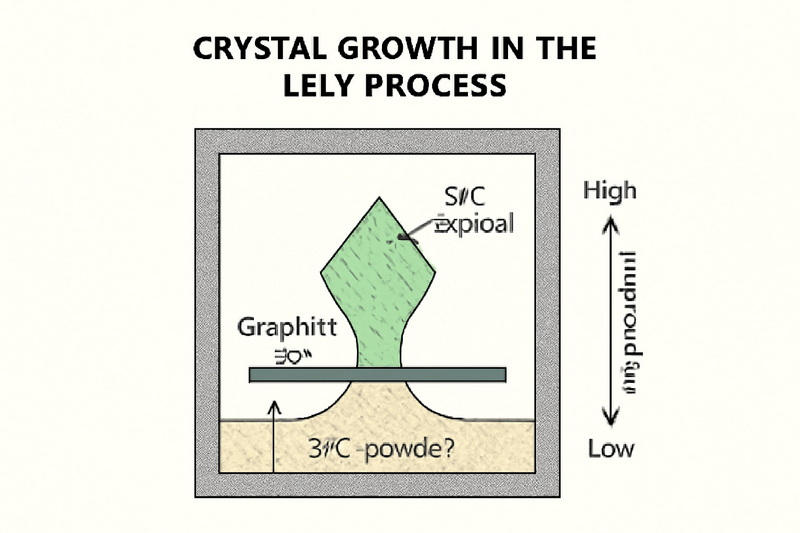
The Lely and Modified Lely Methods
The Lely process focuses on growing high-purity SiC single crystals. Sublimation of SiC powder at 2,700°C deposits vapor onto a cooler graphite rod, forming large crystals. Modern adaptations use induction heating and precise temperature gradients to grow 4-inch-diameter crystals, crucial for semiconductor applications.
Chemical Vapor Deposition (CVD)
CVD generates ultra-pure SiC layers by reacting silane, hydrogen, and nitrogen gases on a substrate. This method enables precise control over crystal structure and doping, producing materials for high-voltage electronics and radiation-resistant sensors.
Sustainable Production Innovations
Emerging methods, such as Susteon's methane-based process, convert recycled silicon waste into β-SiC at 75% lower CO₂ emissions. By leveraging biogas and fugitive methane, this approach reduces costs to $10–$20/kg, offering a greener pathway for the production of silicon carbide.
Crystal Structure and Polymorphs of Silicon Carbide
Silicon carbide exists in more than 200 crystalline forms, known as polytypes, each with unique stacking sequences of silicon and carbon atoms. The most common polytypes are 3C-SiC (cubic), 4H-SiC, and 6H-SiC (hexagonal). These variations influence the material's electrical, thermal, and mechanical properties, making SiC highly versatile for different applications. For instance, 4H-SiC is preferred in high-power electronics due to its wide bandgap and high electron mobility.
Raw Material Preparation and Processing
1. Sourcing and Purification
High-purity silica sand and carbonaceous materials undergo chemical or thermal purification to remove contaminants like iron oxides.
2. Mixing and Reaction
Raw materials are blended in precise ratios and heated in electric arc furnaces. The carbothermic reaction yields crude SiC, which is cooled and crushed into powder.
3. Sintering and Shaping
SiC powder is mixed with sintering aids (e.g., boron or aluminum) and formed via pressing, extrusion, or casting. Sintering at 2,000–2,600°C produces dense, near-net-shape components.
Industrial Applications of Silicon Carbide
- Abrasives and Cutting Tools: SiC's hardness (29 GPa) makes it ideal for grinding wheels and sandblasting media.
- Semiconductors: SiC wafers enable efficient power electronics for EVs and renewable energy systems.
- High-Temperature Components: SiC crucibles and kiln furniture withstand molten metals and ceramic sintering.
- Defense: Armor plates and missile nose cones leverage SiC's lightweight and ballistic resistance.
Silicon Carbide in Emerging Technologies
Beyond traditional uses, silicon carbide is gaining attention in cutting-edge fields. In quantum computing, SiC's ability to host stable quantum bits (qubits) at room temperature offers promising avenues for scalable quantum devices. Additionally, its robustness and thermal stability make it ideal for space exploration components exposed to extreme radiation and temperature fluctuations.
Production Challenges and Innovations
Despite advances, producing high-quality silicon carbide remains challenging. Controlling defects during crystal growth is critical, as imperfections can affect semiconductor performance. Researchers are developing novel techniques such as seeded growth and advanced doping methods to enhance crystal quality. Moreover, scaling up production while maintaining purity and reducing costs is a continuous focus.
Environmental Impact and Mitigation
Traditional methods like the Acheson process generate significant CO₂ emissions due to high energy consumption. Manufacturers are adopting renewable energy for furnaces, recycling waste silicon, and optimizing reaction efficiency. Methane-based synthesis reduces reliance on fossil fuels, aligning production of silicon carbide with circular economy principles.
Environmental Regulations and Sustainability Efforts
The silicon carbide industry faces increasing pressure to comply with stringent environmental regulations. Companies are investing in cleaner production technologies, waste recycling, and energy-efficient furnaces. Life cycle assessments are becoming standard practice to minimize environmental footprints, ensuring that silicon carbide production aligns with global sustainability goals.
Market Dynamics and Scalability
Global demand for SiC is driven by electric vehicles, 5G infrastructure, and renewable energy projects. Scaling production requires addressing challenges like maintaining crystal purity and reducing costs. Automated process controls and AI-driven quality monitoring are being implemented to enhance yield and consistency.
Future Trends
Research focuses on growing larger SiC crystals (up to 8 inches) for semiconductors and hybrid methods combining CVD with 3D printing. Recycling initiatives aim to repurpose industrial silicon waste into high-grade SiC, further optimizing the production of silicon carbide.
Final Thoughts on Silicon Carbide Production
As the demand for silicon carbide continues to grow across various high-tech industries, the production processes are evolving to meet these needs sustainably and efficiently. Innovations in crystal growth, environmental management, and application development ensure that silicon carbide remains a critical material for future technological advancements.
Conclusion
The production of silicon carbide has evolved from the energy-intensive Acheson process to advanced, sustainable methods. As industries demand higher performance and lower environmental impact, innovations in recycling and crystal growth will drive SiC's adoption in next-generation technologies.

FAQs
1. What raw materials are essential for silicon carbide production?
High-purity silica sand and carbon sources like petroleum coke form the basis of traditional SiC synthesis.
2. How does the Acheson process differ from the Lely method?
The Acheson process produces polycrystalline SiC for industrial use, while the Lely method grows single crystals for electronics.
3. Can silicon carbide be recycled during production?
Yes, emerging methods repurpose silicon waste and methane to reduce costs and emissions in the production of silicon carbide.
4. What industries benefit most from reaction-bonded SiC?
RBSC's complex shapes and high strength suit aerospace, automotive, and precision engineering applications.
5. How do sintering additives improve SiC quality?
Additives like boron enhance densification during sintering, improving mechanical and thermal properties.














