Content Menu
● Introduction to Tungsten Carbide WC
● The Chemistry and Structure of Tungsten Carbide
>> Chemical Composition
>> Atomic Arrangement
● Physical and Mechanical Properties
>> Hardness and Strength
>> Density and Thermal Stability
>> Corrosion and Wear Resistance
>> Toughness and Fracture Resistance
● Manufacturing Process of Tungsten Carbide
>> Raw Material Preparation
>> Carburization
>> Milling and Sieving
>> Compaction and Sintering
>> Finishing
● Major Applications Across Industries
>> Cutting Tools and Machining
>> Mining and Drilling
>> Aerospace and Automotive
>> Oil and Gas
>> Jewelry and Consumer Goods
>> Electronics and Electrical Engineering
>> Medical Devices
● Advantages Over Traditional Materials
>> Comparison with Ceramics and Other Superhard Materials
● Environmental and Economic Considerations
>> Resource Management
>> Recycling
>> Economic Impact
● Innovations and Future Trends in Tungsten Carbide
>> Advanced Coatings and Composites
>> Additive Manufacturing
>> Sustainable Production
>> Expanding Applications
● Conclusion
● Frequently Asked Questions (FAQ)
>> 1. What makes tungsten carbide so hard?
>> 2. How is tungsten carbide different from pure tungsten?
>> 3. Can tungsten carbide be machined or shaped after sintering?
>> 4. Is tungsten carbide safe for use in jewelry and consumer products?
>> 5. What are the main limitations of tungsten carbide?
Tungsten carbide (WC) is one of the most extraordinary and versatile materials in modern technology, engineering, and manufacturing. Known for its exceptional hardness, durability, and resistance to extreme environments, tungsten carbide is a cornerstone of countless industries, from aerospace and mining to jewelry and electronics. This comprehensive article delves into the science, manufacturing, applications, advantages, and future prospects of tungsten carbide, complete with visual descriptions and detailed explanations.
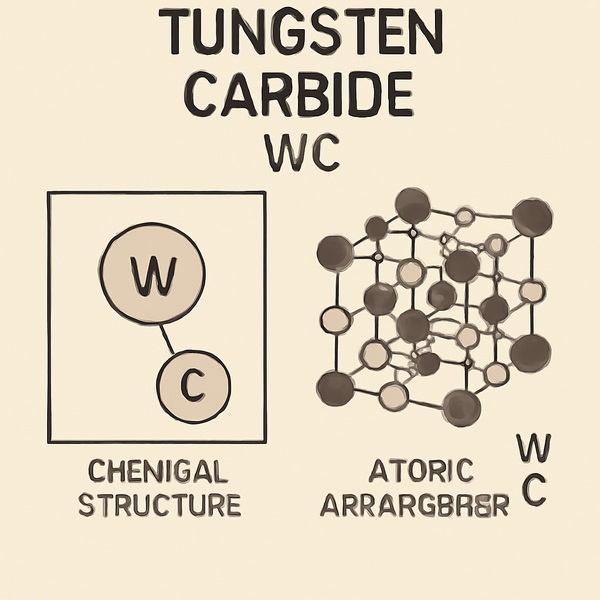
Introduction to Tungsten Carbide WC
Tungsten carbide, abbreviated as WC, is a compound composed of equal parts tungsten and carbon atoms. In its raw form, it appears as a fine, gray powder. Through sophisticated processing, this powder becomes a dense, robust material that underpins high-performance tools, machinery, and even luxury goods.
Imagine a material that can slice through steel, withstand the heat of jet engines, and retain a mirror-like polish for decades. That is the world of tungsten carbide—a material that has transformed the way we build, cut, and create.
The Chemistry and Structure of Tungsten Carbide
Chemical Composition
Tungsten carbide's chemical formula is WC, representing a one-to-one ratio of tungsten (W) and carbon (C) atoms. This compound forms a hexagonal crystal structure, which is fundamental to its remarkable hardness and stability. In industrial settings, WC is usually combined with metallic binders such as cobalt or nickel, resulting in cemented carbides. These composites further enhance toughness and expand the material's versatility.
Atomic Arrangement
The hexagonal lattice of WC is tightly packed, with each tungsten atom surrounded by six carbon atoms. This dense atomic arrangement is key to the material's resistance to deformation and its ability to maintain integrity under extreme pressure and heat. The strong covalent bonds between tungsten and carbon atoms give WC its legendary hardness and durability.
Physical and Mechanical Properties
Hardness and Strength
Tungsten carbide is celebrated for its hardness, ranking about 9 on the Mohs scale—just below diamond. Its Vickers hardness is typically around 2600 HV, and its Young's modulus (a measure of stiffness) ranges from 530 to 700 GPa. This makes it two to three times stiffer than steel, allowing it to maintain sharp edges and resist wear in demanding applications.
Density and Thermal Stability
With a density of approximately 15.6 g/cm³, WC is nearly as dense as gold and about twice as dense as steel. Its melting point is a remarkable 2,780°C, and it maintains its structural stability at high temperatures. This makes it ideal for applications requiring resistance to heat and thermal shock, such as cutting tools and engine components.
Corrosion and Wear Resistance
Tungsten carbide resists oxidation at room temperature and is largely unreactive to most acids, except for aggressive mixtures like hydrofluoric and nitric acid. Its wear resistance is legendary, allowing it to outlast traditional tool materials by a significant margin. This property is especially valuable in environments where abrasion, erosion, and chemical exposure are constant threats.
Toughness and Fracture Resistance
While WC is extremely hard, it is also relatively brittle compared to metals. The addition of metallic binders like cobalt helps to improve its toughness, making it less prone to cracking or shattering under impact. This balance of hardness and toughness is what makes cemented carbide composites so valuable in industrial applications.
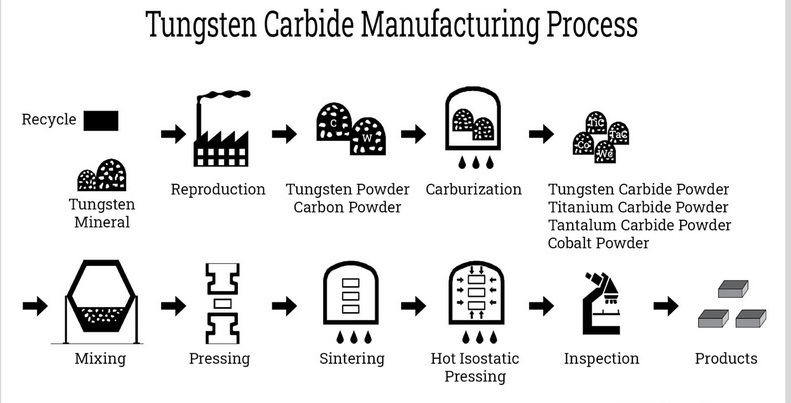
Manufacturing Process of Tungsten Carbide
Raw Material Preparation
The journey of tungsten carbide begins with the selection of high-purity tungsten and carbon sources. Tungsten powder is mixed with carbon black in precise ratios, ensuring uniformity for optimal reaction and final product quality.
Carburization
The mixture is heated in a graphite furnace at temperatures between 1300°C and 1600°C. The intense heat causes the tungsten and carbon to react, forming tungsten carbide powder. This process is carefully controlled to achieve the desired particle size and purity.
Milling and Sieving
The resulting WC powder is milled to achieve the desired particle size distribution and then sieved to ensure uniformity. Fine control over particle size is crucial for the subsequent compaction and sintering steps, as it affects the final material's density and mechanical properties.
Compaction and Sintering
The WC powder is compacted into the required shapes using high-pressure presses. It is then sintered at around 1500°C, often with a metallic binder such as cobalt. Sintering fuses the particles into a dense, solid mass, and the binder helps to improve toughness and resistance to fracture.
Finishing
The sintered components are ground and polished to precise tolerances. Advanced grinding techniques using diamond wheels are necessary due to the material's extreme hardness. The finished products are then inspected for quality and performance, ready for use in a wide range of demanding applications.
Major Applications Across Industries
Cutting Tools and Machining
Tungsten carbide's primary use is in the manufacture of cutting tools—drill bits, end mills, saw blades, and inserts. Its ability to maintain a sharp edge and resist wear makes it indispensable in metalworking, woodworking, and mining operations. For example, a tungsten carbide-tipped saw blade can cut through steel or hardwood with ease, maintaining its sharpness far longer than conventional steel blades.
Mining and Drilling
WC is a critical material in mining and drilling equipment, such as rock drill bits, tunnel boring machines, and excavation tools. The extreme hardness and wear resistance of tungsten carbide allow these tools to cut through rock, concrete, and other hard materials without rapid wear or failure.
Aerospace and Automotive
Aerospace turbines, compressor seals, fuel injector nozzles, and automotive components all benefit from WC's wear and heat resistance. In jet engines, for example, tungsten carbide coatings protect components from high-temperature erosion, ensuring reliability and longevity in critical systems.
Oil and Gas
Drill bits, valve components, and flow control devices in the oil and gas sector are often coated with tungsten carbide to withstand abrasive and corrosive environments. This extends the service life of equipment and reduces maintenance costs in harsh operating conditions.
Jewelry and Consumer Goods
Tungsten carbide's hardness and ability to take a high polish have made it popular in jewelry, especially rings and watch bands. These items are prized for their scratch resistance, weight, and enduring luster. Tungsten carbide jewelry is often chosen for its modern, industrial aesthetic and its ability to withstand daily wear.
Electronics and Electrical Engineering
WC is used in electrical contacts, heat sinks, and components that require high thermal and electrical conductivity. Its stability under high temperatures and resistance to wear make it ideal for demanding electronic applications.
Medical Devices
In the medical field, tungsten carbide is used for surgical instruments, dental tools, and prosthetic devices. Its biocompatibility and ability to maintain sharp edges make it invaluable in precision medical procedures.
Advantages Over Traditional Materials
| Property | Tungsten Carbide | Steel | High-Speed Steel |
| Hardness (Mohs) | 9 | 4–4.5 | 7.5–8 |
| Density (g/cm³) | 15.6 | 7.8 | 8.0 |
| Melting Point (°C) | 2,780 | 1,370 | 1,400 |
| Wear Resistance | Excellent | Moderate | Good |
| Thermal Stability | Excellent | Moderate | Good |
| Cost | Higher | Lower | Moderate |
Tungsten carbide outperforms traditional tool steels and high-speed steels in hardness, wear resistance, and thermal stability. Although it is more expensive, its longer lifespan and reduced maintenance offset the initial cost for many industrial users.
Comparison with Ceramics and Other Superhard Materials
While ceramics such as alumina and silicon carbide are also hard and wear-resistant, tungsten carbide offers a unique balance of hardness and toughness, especially when combined with metallic binders. This makes it less brittle and more suitable for impact and heavy-duty applications than most ceramics.
Environmental and Economic Considerations
Resource Management
Tungsten is a finite resource, and the mining and processing of tungsten ores require significant energy and environmental management. However, the long service life of WC products reduces the frequency of replacements, contributing to sustainability in industrial operations.
Recycling
WC scrap is highly valuable and is routinely recycled, reducing the need for new raw material extraction and minimizing environmental impact. The recycling process involves reclaiming tungsten and cobalt from used tools and reprocessing them into new products.
Economic Impact
The high initial cost of tungsten carbide is offset by its durability and reduced downtime for tool replacement. Industries that rely on WC often experience lower total operating costs and improved productivity, making it a sound investment despite the higher upfront expense.
Innovations and Future Trends in Tungsten Carbide
Advanced Coatings and Composites
Recent innovations include the development of nanostructured WC composites and advanced coatings that further enhance wear resistance, toughness, and corrosion resistance. These materials are being used in aerospace, defense, and energy sectors to push the boundaries of performance.
Additive Manufacturing
Additive manufacturing, or 3D printing, is beginning to make inroads into the production of tungsten carbide components. This technology allows for complex geometries and customized solutions that were previously impossible with traditional manufacturing methods.
Sustainable Production
Efforts are underway to make tungsten carbide production more sustainable, including the use of recycled materials, energy-efficient processes, and environmentally friendly binders. These initiatives aim to reduce the environmental footprint of WC while maintaining its superior performance.
Expanding Applications
As technology advances, new applications for tungsten carbide continue to emerge. In the renewable energy sector, for example, WC is being used in wind turbine components and solar panel manufacturing equipment. Its unique properties make it a material of choice for the next generation of high-performance, sustainable technologies.
Conclusion
Tungsten carbide WC is a material that has revolutionized modern industry with its unparalleled combination of hardness, wear resistance, and thermal stability. From cutting tools that shape the world's infrastructure to jewelry that endures for generations, WC's versatility and performance are unmatched. As technology advances and demands for durability and efficiency grow, tungsten carbide will continue to play a pivotal role in shaping the future of manufacturing, engineering, and design. With ongoing innovations in production, recycling, and application, the story of tungsten carbide is far from over—it is a material that will remain at the forefront of progress for decades to come.

Frequently Asked Questions (FAQ)
1. What makes tungsten carbide so hard?
Tungsten carbide's hardness results from its strong covalent bonds between tungsten and carbon atoms within a dense hexagonal crystal structure. This atomic arrangement resists deformation and abrasion, making WC nearly as hard as diamond.
2. How is tungsten carbide different from pure tungsten?
While pure tungsten is a ductile metal, tungsten carbide is a ceramic-like compound formed by combining tungsten with carbon. This transformation imparts extreme hardness, wear resistance, and brittleness, which are not present in pure tungsten.
3. Can tungsten carbide be machined or shaped after sintering?
Tungsten carbide is extremely difficult to machine after sintering due to its hardness. Specialized diamond or cubic boron nitride tools are required for grinding and shaping. Most WC components are formed to near-net shape before sintering to minimize post-processing.
4. Is tungsten carbide safe for use in jewelry and consumer products?
Yes, tungsten carbide is chemically stable and hypoallergenic, making it safe for use in jewelry. However, due to its brittleness, WC rings can crack or shatter under severe impact, so care should be taken.
5. What are the main limitations of tungsten carbide?
While WC excels in hardness and wear resistance, it is relatively brittle compared to metals. It can fracture under high impact or shock loads. Additionally, its high density makes it heavier than alternative materials, which may be a consideration in some applications.
















