Content Menu
● Introduction to Tungsten Carbide
● Chemical Formula and Structure
>> Atomic Structure
>> Other Tungsten Carbides
● Physical and Chemical Properties
>> Key Physical Properties
>> Chemical Properties
>> Mechanical Properties
>> Thermal and Electrical Properties
● How is Tungsten Carbide Made?
>> 1. Raw Materials
>> 2. Reduction and Carburization
>> 3. Powder Metallurgy and Sintering
>> 4. Shaping and Finishing
>> 5. Quality Control
● Applications of Tungsten Carbide
>> Industrial Applications
>> Specialized Uses
>> Everyday Life
● Advantages and Limitations
>> Advantages
>> Limitations
● History and Development
● Environmental Impact and Recycling
>> Environmental Concerns
>> Recycling
● Future Trends in Tungsten Carbide
● Conclusion
● FAQ: Tungsten Carbide
>> 1. What is the chemical formula for tungsten carbide?
>> 2. How does tungsten carbide compare to steel in terms of hardness?
>> 3. What are the main industrial uses of tungsten carbide?
>> 4. How is tungsten carbide produced?
>> 5. Is tungsten carbide recyclable?
● Citations:
Tungsten carbide is a material renowned for its exceptional hardness, durability, and wide range of industrial applications. But what exactly is tungsten carbide, and what is its chemical formula? In this comprehensive article, we will explore the chemical nature, synthesis, properties, applications, history, environmental impact, and frequently asked questions about tungsten carbide. Along the way, you will find numerous illustrations and diagrams to enhance your understanding of this fascinating compound.

Introduction to Tungsten Carbide
Tungsten carbide is a compound composed of tungsten and carbon atoms in equal proportions. It is most commonly encountered as a fine gray powder, but it can be pressed and sintered into various solid shapes for industrial use. Its outstanding hardness and resistance to wear make it indispensable in manufacturing, mining, construction, and even jewelry.
Chemical Formula and Structure
The chemical formula for tungsten carbide is WC. This formula indicates that each molecule contains one tungsten (W) atom and one carbon (C) atom.
Atomic Structure
- Tungsten (W): Atomic number 74, a transition metal known for its high melting point.
- Carbon (C): Atomic number 6, a nonmetal that forms diverse compounds.
In tungsten carbide, these atoms are arranged in a hexagonal crystal structure at room temperature (α-WC), though a cubic form (β-WC) can exist at high temperatures.
Other Tungsten Carbides
While WC is the most common and commercially significant form, another compound, tungsten semicarbide (W₂C), also exists but is less widely used. W₂C has a different stoichiometry and slightly different properties but is not as hard or as widely applied as WC.
Physical and Chemical Properties
Tungsten carbide's properties make it one of the most valuable materials in modern industry.
Key Physical Properties
| Property | Value |
| Chemical Formula | WC |
| Molar Mass | 195.85 g/mol |
| Crystal Structure | Hexagonal |
| Density | 15.6 g/cm³ |
| Melting Point | 2,870°C (5,198°F) |
| Boiling Point | 6,000°C (10,832°F) |
| Mohs Hardness | 9–9.5 |
| Young's Modulus | 530–700 GPa |
| Thermal Conductivity | 110 W/(m·K) |
| Electrical Resistivity | 0.2 μΩ·m |
Chemical Properties
- Insoluble in water, hydrochloric acid, and sulfuric acid.
- Soluble in a mixture of nitric acid and hydrofluoric acid.
- Begins to oxidize in air at about 500–600°C.
- Reacts with chlorine above 400°C and with fluorine even at room temperature.
Mechanical Properties
Tungsten carbide is valued for its combination of high compressive strength, stiffness, and resistance to deformation. It can withstand enormous forces without bending or breaking, making it ideal for applications involving high pressure or impact.
Thermal and Electrical Properties
Tungsten carbide exhibits good thermal conductivity, allowing it to dissipate heat efficiently during cutting or drilling operations. It also has low electrical resistivity, which is useful in certain electronic and electrical applications.
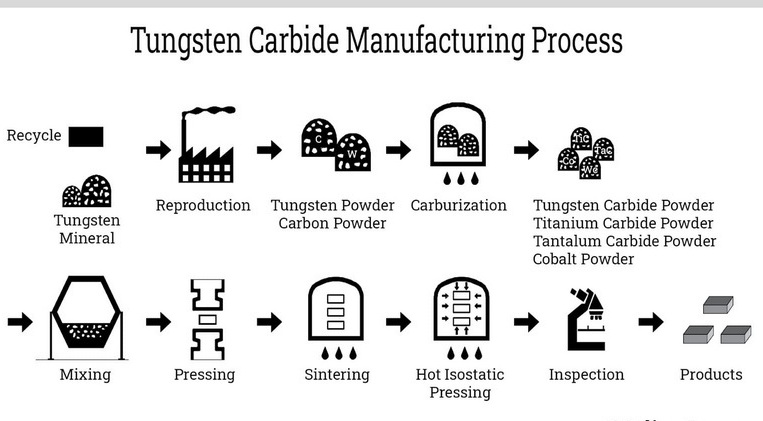
How is Tungsten Carbide Made?
The synthesis of tungsten carbide involves several steps, primarily relying on powder metallurgy.
1. Raw Materials
- Tungsten Ore: Processed into ammonium paratungstate (APT), then tungsten oxide.
- Carbon Source: Graphite or carbon black.
2. Reduction and Carburization
- Tungsten oxide is reduced to metallic tungsten powder in a hydrogen atmosphere.
- The tungsten powder is mixed with carbon and heated to 1,400–2,000°C, forming WC through carburization.
W+C→WC
3. Powder Metallurgy and Sintering
- The WC powder is mixed with a binder, typically cobalt, to enhance toughness.
- The mixture is pressed into shape and sintered at high temperatures (1,400–1,600°C), where the binder melts and cements the particles together.
4. Shaping and Finishing
After sintering, tungsten carbide parts may undergo grinding, lapping, or polishing to achieve precise dimensions and surface finishes. Due to its hardness, only diamond or cubic boron nitride tools can effectively machine tungsten carbide.
5. Quality Control
Finished products are rigorously inspected for density, hardness, microstructure, and dimensional accuracy to ensure they meet stringent industry standards.
Applications of Tungsten Carbide
Tungsten carbide's unique combination of hardness, strength, and chemical stability makes it invaluable across many sectors.
Industrial Applications
- Cutting Tools: Drill bits, milling cutters, saw blades, and lathe tools.
- Mining and Construction: Rock drill bits, excavation tools, and wear-resistant parts.
- Aerospace: Engine components and turbine blades.
- Oil and Gas: Drilling equipment and valves.
- Electronics: Precision components and contacts.
- Jewelry: Rings and watches valued for their scratch resistance and luster.
Specialized Uses
- Armor-piercing ammunition: Due to its density and hardness, tungsten carbide is used in military projectiles.
- Surgical instruments: Some surgical tools are made from tungsten carbide for precision and durability.
- Nuclear technology: Tungsten carbide is used in control rods and shielding materials due to its stability under radiation.
Everyday Life
Tungsten carbide is also found in everyday objects such as fishing weights, sports equipment, and even in some high-end pens.
Advantages and Limitations
Advantages
- Extreme Hardness: Second only to diamond on the Mohs scale.
- Wear Resistance: Retains sharpness and resists abrasion.
- High Melting Point: Suitable for high-temperature applications.
- Corrosion Resistance: Stable in most environments.
- Dimensional Stability: Maintains shape under heavy loads and high temperatures.
Limitations
- Brittleness: Can fracture under extreme impact or stress.
- Difficult to Machine: Requires diamond or cubic boron nitride tools for shaping.
- Cost: More expensive than steel or other common tool materials.
- Weight: Its high density makes it heavier than many alternative materials.
History and Development
The history of tungsten carbide dates back to the early 20th century. In 1923, German company Krupp developed the first practical method for producing cemented carbide (WC with a cobalt binder), revolutionizing the tool-making industry. This innovation enabled the mass production of high-speed cutting tools, which dramatically improved manufacturing efficiency and precision.
Over the decades, advances in powder metallurgy, binder chemistry, and sintering technology have further enhanced the performance and versatility of tungsten carbide. Today, it is a critical material in industries ranging from mining to aerospace.
Environmental Impact and Recycling
Environmental Concerns
The extraction and processing of tungsten ore can have significant environmental impacts, including habitat disruption, water pollution, and energy consumption. The use of cobalt as a binder also raises ethical and environmental questions, as cobalt mining is associated with human rights and ecological issues in some regions.
Recycling
Fortunately, tungsten carbide is highly recyclable. Scrap tools and components can be collected, crushed, and chemically processed to recover tungsten and cobalt for reuse. Recycling not only conserves valuable resources but also reduces the environmental footprint of tungsten carbide production.
Recycling process:
1. Collection of scrap carbide materials.
2. Crushing and milling into fine powder.
3. Chemical treatment to separate tungsten and cobalt.
4. Purification and reuse in new products.
Future Trends in Tungsten Carbide
As technology advances, the demand for materials with superior performance characteristics continues to grow. Researchers are exploring new binder materials, nano-structured carbides, and composite systems to further enhance the toughness, wear resistance, and versatility of tungsten carbide.
Emerging applications include:
- Additive manufacturing (3D printing) of tungsten carbide components.
- Coatings for cutting tools to extend service life.
- Advanced electronics and micro-mechanical systems (MEMS).
The ongoing development of sustainable mining and recycling practices will also play a crucial role in the future of tungsten carbide.
Conclusion
Tungsten carbide, with the chemical formula WC, stands as a cornerstone material in modern industry due to its unparalleled hardness, durability, and resistance to wear and heat. Its synthesis through advanced powder metallurgy techniques and its ability to be tailored with binders like cobalt have made it indispensable for cutting tools, mining equipment, aerospace, electronics, and even jewelry. While it does have some limitations, notably its brittleness and cost, the benefits far outweigh the drawbacks for many demanding applications. As technology advances, tungsten carbide will undoubtedly continue to play a vital role in shaping the tools and components that drive progress across industries.

FAQ: Tungsten Carbide
1. What is the chemical formula for tungsten carbide?
The chemical formula for tungsten carbide is WC, indicating a 1:1 ratio of tungsten and carbon atoms.
2. How does tungsten carbide compare to steel in terms of hardness?
Tungsten carbide is significantly harder than steel, ranking 9–9.5 on the Mohs scale, while most steels are around 4–5. This makes tungsten carbide much more wear-resistant and suitable for cutting and drilling applications.
3. What are the main industrial uses of tungsten carbide?
Tungsten carbide is widely used for cutting tools, mining and drilling equipment, wear-resistant machine parts, aerospace components, and jewelry due to its exceptional hardness and durability.
4. How is tungsten carbide produced?
Tungsten carbide is produced by reacting tungsten metal powder with carbon at high temperatures (1,400–2,000°C), followed by powder metallurgy processes such as mixing with a binder and sintering to form solid shapes.
5. Is tungsten carbide recyclable?
Yes, tungsten carbide is recyclable. Scrap and worn-out tools can be processed to recover the valuable tungsten and carbon, reducing waste and conserving resources.
Citations:
[1] https://en.wikipedia.org/wiki/Tungsten_carbide
[2] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[3] https://heegermaterials.com/blog/90_how-is-tungsten-carbide-made-.html
[4] https://www.istockphoto.com/photos/tungsten-carbide
[5] https://www.retopz.com/57-frequently-asked-questions-faqs-about-tungsten-carbide/
[6] https://www.refractorymetal.org/tungsten-carbide-uses-properties.html
[7] https://www.wj-tool.com/material
[8] https://www.carbide-part.com/blog/tungsten-carbide-materials-characteristics-advantages-and-comprehensive-applications-analysis/
[9] https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2174365.htm
[10] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[11] https://shop.machinemfg.com/the-pros-and-cons-of-tungsten-carbide-a-comprehensive-guide/
[12] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[13] https://softschools.com/formulas/chemistry/tungsten_iv_carbide_formula/462/
[14] https://pubchem.ncbi.nlm.nih.gov/compound/tungsten_carbide
[15] https://echa.europa.eu/registration-dossier/-/registered-dossier/15382
[16] https://www.thermofisher.com/order/catalog/product/tw/zt/012482.22
[17] https://www.hitechseals.com/includes/pdf/tungsten_carbide.pdf
[18] https://grafhartmetall.com/en/what-is-tungsten-carbide/
[19] https://www.harcourt.co/overview_documents/Tungsten%20Carbide%20data%20sheet.PDF
[20] https://www.chemicalbull.com/products/tungsten-carbide
[21] https://www.imetra.com/tungsten-carbide-material-properties/
[22] https://pubchem.ncbi.nlm.nih.gov/compound/Tungsten-carbide
[23] https://preview.fishersci.no/shop/products/tungsten-carbide-99-5-metals-basis-alfa-aesar-2/p-4904562
[24] https://www.azom.com/properties.aspx?ArticleID=1203
[25] https://rrcarbide.com/understanding-tungsten-carbide-composition-uses-and-expertise/
[26] https://zh.wikipedia.org/zh-cn/%E7%A2%B3%E5%8C%96%E9%8E%A2
[27] https://stock.adobe.com/search?k=tungsten+carbide
[28] https://www.shutterstock.com/search/tungsten
[29] https://commons.wikimedia.org/wiki/File:-Alpha_tungsten_carbide_crystal_structure.jpg
[30] https://www.alamy.com/stock-photo/tungsten-carbide.html
[31] https://www.freepik.com/free-photos-vectors/tungsten-carbide
[32] https://www.basiccarbide.com/tungsten-carbide-grade-chart/
[33] https://scienceinfo.com/tungsten-carbide-properties-applications/
[34] https://www.shutterstock.com/search/tungsten-carbide
[35] https://stock.adobe.com/search?k=carbide
[36] https://www.dymetalloys.co.uk/what-is-tungsten-carbide/grade-chart
[37] https://next-gen.materialsproject.org/materials/mp-1894
[38] https://www.gettyimages.hk/%E5%9C%96%E7%89%87/tungsten-carbide
[39] https://theartisanrings.com/pages/tungsten-rings-faqs
[40] https://www.tungstenringsco.com/faq
[41] https://www.tungstenrepublic.com/Tungsten-Carbide-Rings-FAQ.html
[42] https://eternaltools.com/blogs/tutorials/tungsten-carbide-an-informative-guide
[43] https://www.hit-tw.com/newsdetails.aspx?nid=298
[44] https://www.tungco.com/insights/blog/frequently-asked-questions-used-tungsten-carbide-inserts/
[45] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/use.html
[46] https://unbreakableman.co.za/pages/all-about-tungsten-carbide-faq
[47] https://etrnl.com.au/blogs/news/answering-all-of-your-questions-about-tungsten-rings
[48] https://www.carbidetek.com/faqs/
[49] https://www.ipsceramics.com/wp-content/uploads/2022/01/HSDS-14-Tungsten-Carbide-Issue-1.pdf
[50] https://tuncomfg.com/about/faq/
[51] https://www.sciencedirect.com/science/article/abs/pii/S135063071630276X
[52] https://www.sciencedirect.com/science/article/abs/pii/S1526612520307787
[53] https://www.endmills-wotek.com/en/blog/detail/39
[54] https://www.hdtools.com.tw/application/semiconductor-industry.html
[55] https://echa.europa.eu/substance-information/-/substanceinfo/100.031.918
[56] https://www.mmc-carbide.com/cn/download/magazine/vol03/tec_vol03
[57] https://www.sciencedirect.com/science/article/pii/S1003632620653316
[58] https://www.hdtools.com.tw/application/home-appliance-industry.html
[59] https://www.sciencedirect.com/topics/physics-and-astronomy/tungsten-carbide
[60] https://tapmatic.com/product-line-msds-carbide-stylus-material.ydev
[61] https://www.vedantu.com/chemistry/tungsten-carbide
[62] https://www.gettyimages.hk/%E5%9C%96%E7%89%87/tungsten-carbide?page=2
[63] https://www.istockphoto.com/photos/tungsten-carbide-drill-bits
[64] https://en.wikipedia.org/wiki/File:-Alpha_tungsten_carbide_crystal_structure.jpg
[65] https://create.vista.com/photos/tungsten-carbide/
[66] https://periodictable.com/Elements/074/pictures.html
[67] https://www.totalmateria.com/en-us/articles/tungsten-carbide-metals-1/
[68] https://www.thermalspray.com/questions-tungsten-carbide/
[69] https://powder.samaterials.com/tds/sc/1733388175-DP1931.pdf
[70] https://en.wikipedia.org/wiki/Tungsten_carbide
[71] https://nj.gov/health/eoh/rtkweb/documents/fs/1960.pdf
[72] https://www.skyquestt.com/report/tungsten-carbide-market
[73] https://www.scielo.br/j/mr/a/YkbsBHjCFSWN7xtFbWzcmQj/?lang=en
[74] https://generalcarbide.com/pdf/General-Carbide-Designers-Guide-Tungsten-Carbide.pdf
[75] https://hpvchemicals.oecd.org/ui/handler.axd?id=ed1c76bf-dad9-4baa-8d1b-70fed7f92862
















