Content Menu
● Introduction to Tungsten Carbide
>> Chemical Composition
>> Physical Properties
● Synthesis of Tungsten Carbide
● Manufacturing Process
● Applications of Tungsten Carbide
● Coatings and Surface Treatments
>> HVOF Process
>> D-Gun Process
● Environmental and Health Considerations
● Future Developments
● Conclusion
● FAQs
>> 1. What is the primary use of tungsten carbide?
>> 2. How is tungsten carbide synthesized?
>> 3. What are the common binders used in tungsten carbide products?
>> 4. What are the advantages of tungsten carbide coatings?
>> 5. Is tungsten carbide used in non-industrial applications?
● Citations:
Tungsten carbide is a highly versatile and durable material widely used in various industrial applications, including cutting tools, wear-resistant parts, and even jewelry. The question of whether tungsten carbide is man-made is straightforward: yes, it is synthesized through a process involving tungsten and carbon. This article will delve into the synthesis, properties, applications, and manufacturing processes of tungsten carbide, providing insights into its man-made nature.

Introduction to Tungsten Carbide
Tungsten carbide, with the chemical formula WC, is a compound made from equal parts of tungsten and carbon atoms. It is known for its exceptional hardness, wear resistance, and thermal properties, making it an indispensable material in modern industry.
Chemical Composition
Tungsten carbide typically consists of approximately 94% tungsten and 6% carbon by weight. However, its composition can be modified by adding metallic binders like cobalt or nickel to enhance certain properties. These binders improve the sinterability and toughness of the final product.
Physical Properties
- Hardness and Density: Tungsten carbide is extremely hard, with a density twice that of steel. It is comparable to corundum in hardness and is only surpassed by diamond among common industrial materials.
- Thermal Conductivity: It maintains its structural integrity across a wide temperature range due to its high thermal conductivity, which ensures efficient heat dissipation in high-temperature operations.
- Corrosion Resistance: Tungsten carbide exhibits good resistance to corrosion, especially when compared to other hard metals, making it suitable for use in harsh environments.
Synthesis of Tungsten Carbide
The synthesis of tungsten carbide involves heating tungsten metal or powder with carbon at high temperatures. Here are some common methods:
1. Direct Carburization: Tungsten metal is heated with carbon at temperatures between 1,400°C and 2,000°C.
2. Fluid Bed Process: This method involves reacting tungsten metal or tungsten trioxide with a CO/CO2 mixture and hydrogen at lower temperatures (900°C to 1,200°C).
3. Chemical Vapor Deposition (CVD): Tungsten hexachloride reacts with hydrogen and methane at 670°C to form tungsten carbide.
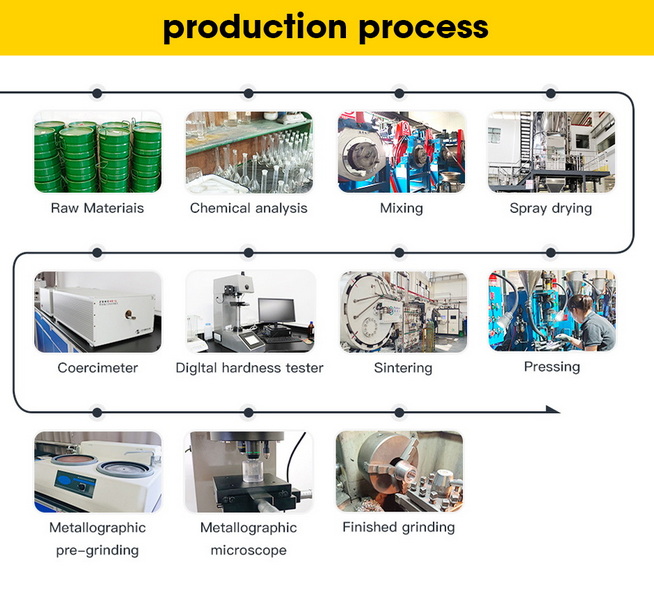
Manufacturing Process
The manufacturing of tungsten carbide products typically involves powder metallurgy techniques:
1. Mixing: Tungsten and carbon powders are mixed.
2. Carburization: The mixture is heated to form tungsten carbide.
3. Grinding and Mixing with Binder: The tungsten carbide powder is ground and mixed with a binder, usually cobalt.
4. Pressing: The mixture is pressed into the desired shape.
5. Sintering: The pressed part is heated to a high temperature (around 1,600°C) to bind the particles together.
Applications of Tungsten Carbide
Tungsten carbide is used in a variety of applications due to its hardness and wear resistance:
- Cutting Tools: Drills, saws, and milling cutters benefit from tungsten carbide tips for their durability and ability to maintain sharpness.
- Wear-Resistant Parts: Used in machinery components that require high wear resistance, such as nozzles and valves.
- Jewelry: Tungsten carbide is also used in jewelry due to its hardness and resistance to scratches.
- Aerospace and Defense: Its high strength-to-weight ratio makes it suitable for components in aircraft and defense equipment.
Coatings and Surface Treatments
Tungsten carbide coatings are applied using advanced techniques like High-Velocity Oxygen Fuel (HVOF) and Detonation Gun (D-Gun) processes. These coatings enhance the wear resistance and durability of components in demanding environments.
HVOF Process
- Description: HVOF spraying uses a high-temperature, high-velocity gas stream to accelerate carbide particles onto a substrate, producing dense coatings with superior wear resistance.
D-Gun Process
- Description: This process uses controlled detonations to accelerate particles to supersonic velocities, creating exceptionally dense and well-bonded coatings.
Environmental and Health Considerations
The production and use of tungsten carbide have environmental and health implications:
- Environmental Impact: The mining of tungsten can have environmental impacts, such as soil and water pollution. Efforts are being made to improve mining practices and reduce waste.
- Health Risks: Exposure to tungsten carbide dust during manufacturing can pose health risks, including respiratory issues. Proper safety measures are essential to mitigate these risks.
Future Developments
Research into new applications and manufacturing techniques for tungsten carbide continues to advance its use in various industries:
- Advanced Materials: The development of new composite materials that incorporate tungsten carbide is expected to enhance its properties further.
- 3D Printing: The integration of tungsten carbide into 3D printing technologies could revolutionize the production of complex shapes and structures.
Conclusion
Tungsten carbide is indeed a man-made material, synthesized through various high-temperature processes involving tungsten and carbon. Its exceptional hardness, wear resistance, and thermal properties make it a crucial component in industrial applications, from cutting tools to wear-resistant parts and even jewelry. The manufacturing process involves powder metallurgy techniques, often combined with metallic binders to enhance its properties.

FAQs
1. What is the primary use of tungsten carbide?
Tungsten carbide is primarily used in cutting tools and wear-resistant parts due to its exceptional hardness and durability.
2. How is tungsten carbide synthesized?
Tungsten carbide is synthesized by heating tungsten metal or powder with carbon at high temperatures, typically between 1,400°C and 2,000°C.
3. What are the common binders used in tungsten carbide products?
Cobalt is the most common binder used in tungsten carbide products, but nickel and iron can also be used.
4. What are the advantages of tungsten carbide coatings?
Tungsten carbide coatings offer superior wear resistance, hardness, and thermal properties, making them ideal for protecting components in demanding environments.
5. Is tungsten carbide used in non-industrial applications?
Yes, tungsten carbide is also used in non-industrial applications, such as jewelry, due to its hardness and resistance to scratches.
Citations:
[1] https://www.britannica.com/science/tungsten-carbide
[2] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[3] https://www.vedantu.com/chemistry/tungsten-carbide
[4] https://www.alamy.com/stock-photo/tungsten-carbide.html
[5] https://www.kovametalli-in.com/manufacturing.html
[6] https://en.wikipedia.org/wiki/Tungsten_carbide
[7] https://www.gettyimages.hk/%E5%9C%96%E7%89%87/tungsten-carbide?page=2
[8] https://www.mmc-carbide.com/sea/technical_information/tec_guide/tec_guide_carbide
[9] https://create.vista.com/photos/tungsten-carbide/
[10] https://www.dymetalloys.co.uk/what-is-tungsten-carbide
[11] https://www.reddit.com/r/askscience/comments/9whr5d/is_tungsten_carbide_an_alloy/
[12] http://www.titaniumkay.com/tungsten-rings/how-tungsten-carbide-rings-are-made/
[13] https://www.gettyimages.hk/%E5%9C%96%E7%89%87/tungsten-carbide
[14] https://www.freepik.com/free-photos-vectors/tungsten
[15] https://www.shutterstock.com/search/tungsten-metal
[16] https://www.istockphoto.com/photos/tungsten-carbide
[17] https://touchwood.biz/blogs/southafrica/what-is-the-difference-between-pure-tungsten-and-tungsten-carbide
[18] https://carbosystem.com/en/tungsten-carbide/
[19] https://nanopartikel.info/en/knowledge/materials/tungsten-carbide/
[20] https://www.linkedin.com/pulse/tungstencarbide-production-process-tungsten-carbide-shijin-lei
















