Content Menu
● Composition and Properties
● Raw Materials for Producing Tungsten Carbide
● Synthesis of Tungsten Carbide
● Grain Size Control
● Cemented Tungsten Carbide
● Applications of Tungsten Carbide
● Advantages of Tungsten Carbide
● Conclusion
● FAQ
>> 1. What is tungsten carbide?
>> 2. How is tungsten carbide synthesized?
>> 3. What are the primary raw materials for producing tungsten carbide?
>> 4. What are the key properties of tungsten carbide?
>> 5. What are the common applications of tungsten carbide?
● Citations:
Tungsten carbide (WC) is a chemical compound comprising tungsten and carbon atoms. It exists as a fine gray powder in its basic form but can be pressed and formed into shapes through sintering for use in industrial machinery. Known for its exceptional hardness, wear resistance, and thermal properties, tungsten carbide is essential for industrial durability and is widely used in various applications, including cutting tools, wear-resistant parts, and coatings.

Composition and Properties
Tungsten carbide (WC) consists of tungsten and carbon atoms arranged in a hexagonal crystal structure. The most common form used in industrial applications contains approximately 94% tungsten and 6% carbon by weight. Its chemical formula is WC, with a molecular weight of 195.85. The specific arrangement of these atoms gives rise to its unique and desirable properties.
Key Properties:
- Hardness: Tungsten carbide has a hardness comparable to diamond, often measured on the Vickers scale with values exceeding 2000 HV. This extreme hardness makes it ideal for applications where resistance to abrasion and penetration is crucial.
- Density: With a density of about 15.6 g/cm3, it is significantly denser than other carbides like silicon carbide (approximately 3.2 g/cm3) and even denser than many steels (around 7.8 g/cm3). This high density contributes to its stability and robustness in demanding environments.
- Strength: It possesses very high strength for a hard and rigid material. Its compressive strength is higher than virtually all melted, cast, or forged metals and alloys, allowing it to withstand significant pressure and deformation.
- Rigidity: Tungsten carbide is two to three times as rigid as steel and four to six times as rigid as cast iron and brass. This rigidity is a crucial factor in applications where dimensional stability and minimal deflection are required, such as precision machining.
- Thermal Properties: It maintains its structural integrity and performance from room temperature to extreme heat, with one of the highest melting points among engineering materials (2780-2830 ℃). This high melting point and resistance to thermal deformation make it invaluable in high-temperature applications.
- Wear Resistance: Tungsten carbide is renowned for its exceptional wear resistance, making it suitable for demanding industrial applications such as cutting tools, where the material is constantly subjected to friction and abrasion.
- Chemical Inertness: It is insoluble in water, hydrochloric acid, and sulfuric acid but soluble in a mixture of nitric acid and hydrofluoric acid. This chemical inertness makes it suitable for use in corrosive environments where other materials would degrade.
Raw Materials for Producing Tungsten Carbide
The production of tungsten carbide involves several key raw materials, each contributing to the final product's properties:
- Tungsten Ore: Black ore, such as wolframite ((Fe,Mn)WO4) and scheelite (CaWO4), is the primary source of tungsten. These ores are mined and processed to extract tungsten-containing compounds.
- Ammonium Paratungstate (APT): A purified chemical compound derived from tungsten ore serves as an intermediate in the production of tungsten metal and tungsten carbide. APT is produced through a series of chemical processes involving leaching, solvent extraction, and crystallization.
- Tungsten Oxide: Produced by calcinating APT at high temperatures, which is then reduced to tungsten metal powder in a hydrogen atmosphere. The calcination process removes ammonia and water, leaving behind tungsten oxide.
- Carbon Sources: Soot or graphite are used to convert tungsten metal powder into tungsten carbide through a high-temperature carburization process. These carbon sources must be of high purity to avoid introducing impurities into the final product.
Synthesis of Tungsten Carbide
Tungsten carbide can be synthesized through several methods, each with its advantages and limitations. These methods are crucial for controlling the quality and properties of the final product.
1.Direct Reaction of Tungsten and Carbon:Tungsten metal (or powder) and carbon are reacted at high temperatures, typically between 1400°C and 2000°C
2.Fluid Bed Process:A lower-temperature fluid bed process reacts either tungsten metal (or powder) or blue WO3 with a CO/CO2 gas mixture and H2 gas between 900°C and 1200°C
3.Reaction of Tungsten Oxide with Graphite:Tungsten trioxide (WO3) is heated with graphite directly at 900°C or in hydrogen at 670°C, followed by carburization in argon at 1000°C
4.Chemical Vapor Deposition (CVD):Tungsten hexachloride is reacted with hydrogen (as a reducing agent) and methane (as the source of carbon) at 670°C (943 K)
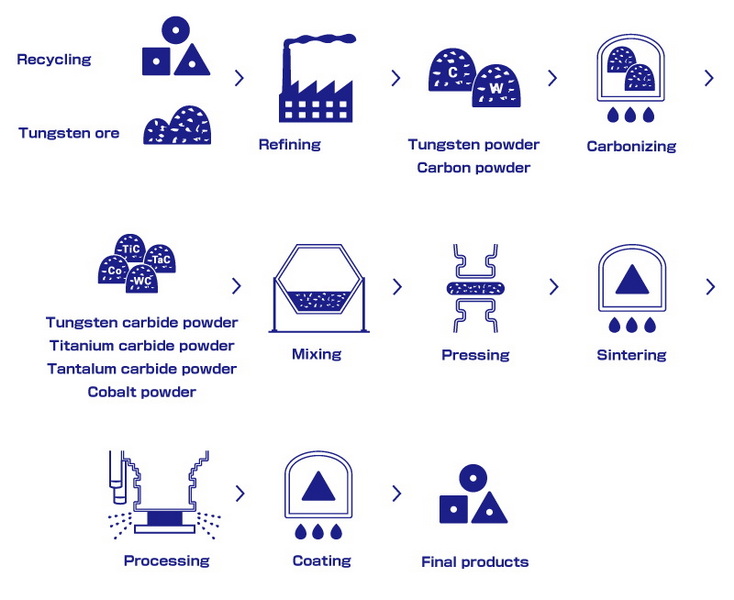
Grain Size Control
The size of the carbide grains significantly determines the mechanical properties of the final product. Finer grain sizes generally lead to higher hardness and strength, while coarser grain sizes may offer improved toughness. The grain size depends on the size of the tungsten oxide particles and the duration and temperature of the oxide/carbon mixture processing. Techniques such as controlling the nucleation rate and employing sintering additives can also be used to influence grain growth.
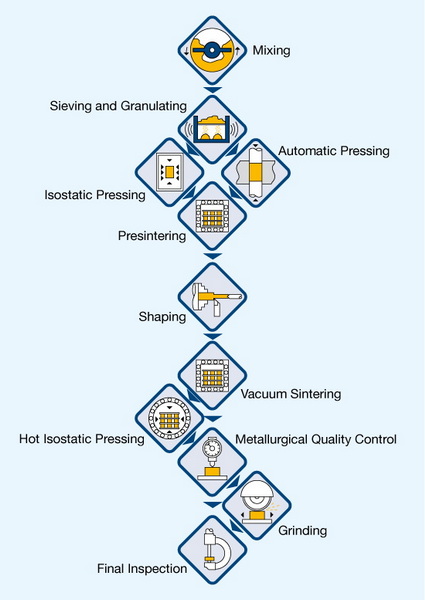
Cemented Tungsten Carbide
To improve its toughness and usability, tungsten carbide is often used in a "cemented" form. This involves binding the tungsten carbide grains together with a metallic binder, typically cobalt. The metallic binder provides ductility and toughness, compensating for the inherent brittleness of tungsten carbide.
Process:
1. Mixing: Powdered tungsten carbide is mixed with a powdered metal binder (usually cobalt, but alternatives include nickel, iron, and paraffin wax). The mixing process is critical to ensure a homogenous distribution of the binder throughout the tungsten carbide matrix.
2. Pressing: The mixture is pressed into the desired shape. Pressing can be done using various techniques, including uniaxial pressing, isostatic pressing, and extrusion, depending on the desired shape and density.
3. Sintering: The pressed compact is then sintered by heating it to temperatures between 1400°C (2550°F) and 1600°C (2910°F). During sintering, the binder melts, wets, and partially dissolves the tungsten carbide grains, binding them together. The sintering process is carried out in a controlled atmosphere to prevent oxidation and maintain the desired microstructure.
4. Result: The resulting composite material, known as cemented carbide, combines the hardness of tungsten carbide with the toughness of the metallic binder. The properties of cemented carbide can be tailored by adjusting the composition, grain size, and sintering parameters.
Applications of Tungsten Carbide
Tungsten carbide's exceptional properties make it suitable for a wide range of applications across various industries. Its hardness, wear resistance, and thermal stability are particularly valued in demanding environments.
- Cutting Tools: Used for high-speed cutting turning tools, milling cutters, drill bits, and inserts. The high hardness and wear resistance of tungsten carbide allow these tools to maintain sharp cutting edges for extended periods, leading to improved machining efficiency and precision.
- Wear-Resistant Parts: Utilized in components that require high wear resistance, such as seals, nozzles, bearings, and dies. These parts are subjected to constant friction and abrasion, and tungsten carbide's superior wear resistance ensures a long service life.
- Kiln Furnace Structural Materials: Employed in high-temperature environments due to its thermal stability and resistance to deformation. Tungsten carbide components can withstand the extreme temperatures and corrosive atmospheres found in industrial furnaces.
- Jet Engine Components: Used in aerospace applications due to its thermal stability and high-temperature strength. Tungsten carbide components can withstand the extreme temperatures and stresses encountered in jet engines, contributing to improved performance and durability.
- Cermet Materials: Used in composite materials combining ceramic and metallic properties. Cermets offer a unique combination of high hardness, wear resistance, and toughness, making them suitable for a variety of demanding applications.
- Resistance Heating Elements: Applied in heating elements due to its electrical conductivity and thermal resistance. Tungsten carbide heating elements can generate high temperatures efficiently and reliably, making them suitable for industrial heating applications.
- Smelting Crucibles: Used for metals such as copper, cobalt, and bismuth. Tungsten carbide crucibles can withstand the high temperatures and corrosive environments involved in metal smelting, ensuring minimal contamination of the molten metal.
- Wear-Resistant Semiconductor Films: Applied in semiconductor manufacturing to protect sensitive components from wear and corrosion. Tungsten carbide films can be deposited using CVD techniques, providing a thin, uniform coating with excellent wear resistance.
- Aerospace Materials: As a modified additive of NbC-C and TaC-C ternary system carbides. Tungsten carbide can enhance the high-temperature strength and oxidation resistance of these composite materials, making them suitable for extreme aerospace applications.
Advantages of Tungsten Carbide
Tungsten carbide offers several advantages that make it a preferred material in many industries. These advantages stem from its unique combination of properties, making it suitable for demanding applications where other materials would fail.
- High Hardness: Provides excellent resistance to wear and abrasion, extending the service life of components and reducing maintenance costs.
- High Strength: Offers superior performance under high-stress conditions, ensuring structural integrity and preventing failure.
- High Rigidity: Ensures minimal deformation and deflection in demanding applications, maintaining dimensional accuracy and precision.
- Thermal Stability: Maintains structural integrity at high temperatures, allowing for use in extreme environments without degradation.
- Chemical Resistance: Resistant to many corrosive substances, ensuring durability and preventing corrosion-related failures.
- Excellent Machinability: Although hard, cemented tungsten carbide can be machined using specialized techniques such as electrical discharge machining (EDM) and grinding, allowing for the creation of complex shapes and precise dimensions.
Conclusion
Tungsten carbide is a versatile and essential material in modern industry, owing to its exceptional hardness, wear resistance, and thermal stability. It is synthesized through various methods, including direct reaction, fluid bed processes, and chemical vapor deposition, each offering unique advantages in terms of control and scalability. Cemented tungsten carbide, combining tungsten carbide with metallic binders, enhances its toughness and applicability, making it suitable for a wide range of demanding applications. Its widespread use in cutting tools, wear-resistant parts, and aerospace components underscores its importance in demanding industrial environments. The ongoing research and development in tungsten carbide materials continue to expand its applications and improve its performance, ensuring its continued relevance in the future.
FAQ
1. What is tungsten carbide?
Tungsten carbide (WC) is a chemical compound consisting of tungsten and carbon atoms. It is known for its exceptional hardness, wear resistance, and thermal stability, making it suitable for various industrial applications. It is often used in the form of cemented carbide, where tungsten carbide grains are bound together by a metallic binder, typically cobalt.
2. How is tungsten carbide synthesized?
Tungsten carbide can be synthesized through several methods, including the direct reaction of tungsten and carbon at high temperatures, fluid bed processes using tungsten oxide, and chemical vapor deposition techniques involving tungsten halides. Each method offers different advantages in terms of control over grain size, purity, and scalability.
3. What are the primary raw materials for producing tungsten carbide?
The primary raw materials include tungsten ore, such as wolframite and scheelite, which are processed to extract tungsten-containing compounds. These compounds are then converted into ammonium paratungstate (APT), tungsten oxide, and finally, tungsten metal powder. Carbon sources, such as soot or graphite, are used to react with the tungsten metal powder to form tungsten carbide.
4. What are the key properties of tungsten carbide?
Key properties include high hardness, often comparable to diamond; high density, making it denser than most metals; high strength, allowing it to withstand significant pressure; high rigidity, ensuring minimal deformation; thermal stability, maintaining its properties at high temperatures; and wear resistance, making it suitable for abrasive environments.
5. What are the common applications of tungsten carbide?
Tungsten carbide is commonly used in cutting tools for machining metals and other materials, wear-resistant parts such as seals and bearings, kiln furnace structural materials for high-temperature environments, jet engine components for aerospace applications, cermet materials combining ceramic and metallic properties, resistance heating elements for industrial heating, smelting crucibles for melting metals, wear-resistant semiconductor films for electronic devices, and aerospace materials for high-performance applications.
Citations:
[1] https://todaysmachiningworld.com/magazine/how-it-works-making-tungsten-carbide-cutting-tools/
[2] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[3] https://scienceinfo.com/tungsten-carbide-properties-applications/
[4] https://en.wikipedia.org/wiki/Tungsten_carbide
[5] https://www.refractorymetal.org/tungsten-carbide-uses-properties.html
[6] https://www.vedantu.com/chemistry/tungsten-carbide
[7] https://repository.up.ac.za/bitstream/handle/2263/24896/03chapter3.pdf?sequence=4
[8] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[9] https://heegermaterials.com/blog/90_how-is-tungsten-carbide-made-.html
















