Content Menu
● Chemical Composition and Properties
● Raw Materials and Production Process
>> 1. Source Materials
>> 2. Manufacturing Steps
● Advanced Manufacturing Techniques
● Applications of Tungsten Carbide
>> 1. Industrial Cutting Tools
>> 2. Mining and Drilling
>> 3. Medical Instruments
>> 4. Consumer Products
● Environmental and Recycling
● Limitations and Solutions
● Conclusion
● FAQs
>> 1. Is tungsten carbide heavier than lead?
>> 2. Can tungsten carbide conduct electricity?
>> 3. How are tungsten carbide coatings applied?
>> 4. What causes carbide tool failure?
>> 5. Are there food-safe tungsten carbide grades?
● Citations:
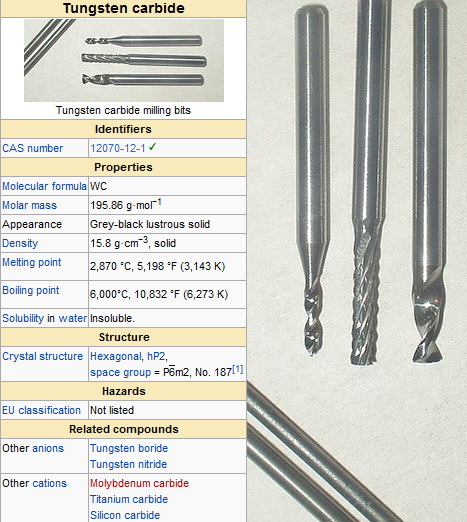
Tungsten carbide (WC) is a synthetic compound renowned for its extreme hardness, wear resistance, and high-temperature stability. Composed of equal parts tungsten and carbon atoms, it's widely used in industrial machinery, cutting tools, mining equipment, and even jewelry. This article explores its composition, manufacturing process, applications, and answers key questions about this remarkable material.

Chemical Composition and Properties
Tungsten carbide consists of tungsten (W) and carbon (C) in a 1:1 atomic ratio, forming a hexagonal crystalline structure with exceptional mechanical properties[34][9]. Key characteristics include:
- Hardness: 8.5–9 on the Mohs scale (nearly as hard as diamond)[1][34].
- Density: ~15.6 g/cm³, making it twice as dense as steel[34].
- Melting point: 2,870°C, one of the highest among industrial materials[9].
- Compressive strength: Exceeds 2.7 GPa, surpassing most metals, including titanium[3][10].
- Young's modulus: 530–700 GPa, providing rigidity 2–3 times greater than steel[3][34].
Suggested image: *Microscopic view of tungsten carbide grains bonded with cobalt (Caption: Tungsten carbide's microstructure under high magnification)[4][36].*
Raw Materials and Production Process
1. Source Materials
- Tungsten ore: Mined as wolframite or scheelite, refined into tungsten oxide (WO₃)[2][35].
- Carbon sources: High-purity graphite or carbon black for carburization[6][8].
- Binders: Cobalt (5–20%) or nickel enhances toughness by filling gaps between WC grains[9][18].
2. Manufacturing Steps
1. Carburization:
Tungsten oxide reacts with carbon at 1,200–1,800°C in hydrogen, forming WC powder[2][8]:
WO3+4C→WC+3CO
2. Mixing and Milling:
WC powder is blended with cobalt in ball mills for 2–4 hours to ensure uniformity[2][6]. Alcohol and paraffin are added to improve flowability during pressing[6][31].
3. Pressing:
The mixture is compacted using hydraulic presses (200–400 MPa) or injection molding[6][18]. Pre-sintering at 600–800°C creates chalk-like "green" parts for CNC machining[6][12].
4. Sintering:
Parts are heated to 1,400–1,600°C in vacuum or argon, causing cobalt to melt and bond WC grains[6][12]. This results in 18% shrinkage and final densities over 14 g/cm³[6][34].
5. Post-Processing:
- Grinding with diamond wheels to achieve ±0.002 mm precision[12].
- Coatings (e.g., titanium nitride) applied via HVOF spraying to enhance thermal stability[17][19].

Advanced Manufacturing Techniques
| Method | Description |
| HIP Treatment | Hot isostatic pressing reduces porosity, improving fatigue resistance637. |
| Additive Manufacturing | Laser powder bed fusion creates complex geometries for aerospace components37. |
| CNC Machining | Pre-sintered "brown" parts are shaped before final sintering612. |
Applications of Tungsten Carbide
1. Industrial Cutting Tools
- Drills and end mills: WC-Co tools cut steel 3x faster than HSS with minimal wear[9][11].
- Lathe inserts: Titanium nitride-coated variants handle temperatures up to 1,100°C[9][19].
Suggested image: *Tungsten carbide end mills machining aerospace alloys[36][32].*
2. Mining and Drilling
- Drill bit tips: WC inserts withstand 250 MPa rock abrasion in oil/gas exploration[14][36].
- Tunnel boring machines: 40% longer lifespan compared to steel teeth[14][36].
3. Medical Instruments
- Surgical blades: Corrosion-resistant WC scalpels maintain sharpness through 500+ incisions[1][11].
- Dental burs: Coated with diamond dust for precision bone sculpting[36][25].
4. Consumer Products
- Jewelry: Scratch-resistant wedding bands polished to mirror finishes[1][14].
- Sports equipment: Golf club inserts increase driving distance by 15%[1][7].
Environmental and Recycling
- Reclamation: 95% of scrap WC is recycled via zinc recovery or direct re-powdering[1][7].
- Energy efficiency: Sintering furnaces use regenerative burners to reduce CO₂ by 30%[37].
Limitations and Solutions
| Challenge | Innovation |
| Brittleness | Gradient sintering with nickel-chromium layers19. |
| High production cost | Cobalt substitution with iron aluminide937. |
| Complex shaping | 3D printing with WC-polymer filaments37. |
Conclusion
Tungsten carbide's dominance in extreme environments stems from its atomic structure and evolving manufacturing techniques. From its 19th-century origins to modern additive manufacturing, WC continues to redefine material limits. As industries demand higher performance, advancements in nano-grained carbides and eco-friendly binders promise to expand its applications into quantum computing and renewable energy systems.
FAQs
1. Is tungsten carbide heavier than lead?
Yes. With a density of 15.6 g/cm³, WC is 40% denser than lead (11.3 g/cm³)[34][16].
2. Can tungsten carbide conduct electricity?
Partially. Its resistivity (0.2 μΩ·m) is comparable to vanadium, enabling use in electrical contacts[9][10].
3. How are tungsten carbide coatings applied?
HVOF (High-Velocity Oxygen Fuel) spraying bonds WC particles at 1,000 m/s for <1% porosity[17][19].
4. What causes carbide tool failure?
Thermal cracking above 800°C or cobalt leaching in acidic coolants[19][34].
5. Are there food-safe tungsten carbide grades?
Yes. Nickel-binded WC (ISO 5832-8) is used in food processing blades[11][14].
Citations:
[1] https://www.tungco.com/insights/blog/5-tungsten-carbide-applications/
[2] https://heegermaterials.com/blog/90_how-is-tungsten-carbide-made-.html
[3] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[4] https://create.vista.com/photos/tungsten-carbide/
[5] https://www.alamy.com/stock-photo/tungsten-carbide-tool.html
[6] https://www.kovametalli-in.com/manufacturing.html
[7] https://www.carbide-usa.com/top-5-uses-for-tungsten-carbide/
[8] https://todaysmachiningworld.com/magazine/how-it-works-making-tungsten-carbide-cutting-tools/
[9] https://en.wikipedia.org/wiki/Tungsten_carbide
[10] https://www.imetra.com/tungsten-carbide-material-properties/
[11] https://www.sollex.se/en/blog/post/about-cemented-tungsten-carbide-applications-part-1
[12] https://www.bangerter.com/en/tungsten-carbide/manufacturing-process
[13] https://www.azom.com/properties.aspx?ArticleID=1203
[14] https://eurobalt.net/blog/2022/03/28/all-the-applications-of-tungsten-carbide/
[15] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/process.html
[16] https://www.dymetalloys.co.uk/what-is-tungsten-carbide
[17] https://www.industrialplating.com/materials/tungsten-carbide-coatings
[18] https://www.psmindustries.com/yillik/tungsten-carbide-manufacturing-process
[19] https://www.asbindustries.com/coating-materials/carbide-coating-materials/tungsten-carbide-coatings
[20] https://generalcarbide.com/pdf/General-Carbide-Designers-Guide-Tungsten-Carbide.pdf
[21] https://www.linkedin.com/pulse/exploring-benefits-tungsten-carbide-tooling-debra-cattle-gqfye
[22] https://www.shutterstock.com/search/tungsten-carbide
[23] https://stock.adobe.com/search?k=tungsten+carbide
[24] https://stock.adobe.com/search?k=tungsten
[25] https://www.istockphoto.com/de/bot-wall?returnUrl=%2Fde%2Fphotos%2Ftungsten-carbide
[26] https://www.shutterstock.com/search/%22tungsten-carbide%22?page=3
[27] https://periodictable.com/Elements/074/pictures.html
[28] https://stock.adobe.com/search?k=carbide
[29] https://www.everloy-cemented-carbide.com/en/process/
[30] https://www.istockphoto.com/de/bot-wall?returnUrl=%2Fde%2Fphotos%2Ftungsten-carbide-drill-bits
[31] https://www.linkedin.com/pulse/tungstencarbide-production-process-tungsten-carbide-shijin-lei
[32] https://www.shutterstock.com/search/solid-tungsten-carbide
[33] https://www.vistametalsinc.com/tungsten-carbide-preform-process/
[34] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[35] https://repository.up.ac.za/bitstream/handle/2263/24896/03chapter3.pdf?sequence=4
[36] https://www.alamy.com/stock-photo/tungsten-carbide.html
[37] https://ceramics.org/ceramic-tech-today/tungsten-carbide-made-easy-government-industry-academia-investigate-additively-manufacturing-cemented-carbide-parts/
[38] https://www.mmc-carbide.com/us/technical_information/tec_guide/tec_guide_carbide
















