Content Menu
● Introduction
● What is Tungsten Carbide?
● The Composition of Tungsten Carbide
● The Sintering Process
● Properties of Tungsten Carbide
● Is Tungsten Carbide an Alloy?
● Types of Tungsten Carbide Composites
● Applications of Tungsten Carbide
● Manufacturing Process
● Advantages and Disadvantages
● Future Developments
● Environmental Considerations
● Conclusion
● Frequently Asked Questions
>> 1. What is the difference between tungsten and tungsten carbide?
>> 2. Can tungsten carbide be recycled?
>> 3. Is tungsten carbide magnetic?
>> 4. How does the hardness of tungsten carbide compare to other materials?
>> 5. Is tungsten carbide safe to wear as jewelry?
● Citations:
Introduction
Tungsten carbide is a remarkable material that has gained significant attention in various industries due to its exceptional properties. However, there is often confusion about whether tungsten carbide should be classified as an alloy or not. This article aims to explore the nature of tungsten carbide, its composition, properties, and applications, to determine its classification and shed light on this intriguing material.
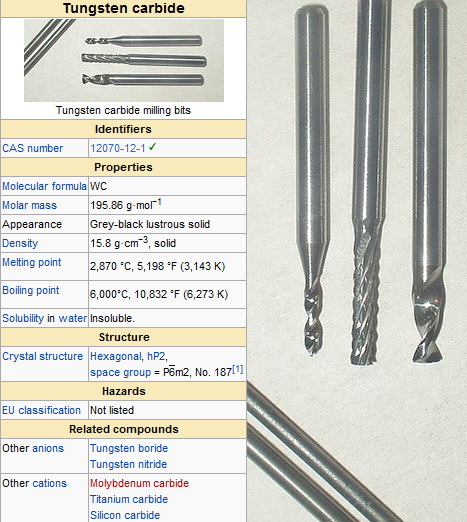
What is Tungsten Carbide?
Tungsten carbide (WC) is a chemical compound composed of equal parts of tungsten and carbon atoms[1]. In its most basic form, it appears as a fine gray powder with a bluish tinge[2]. This compound is renowned for its extreme hardness, high density, and excellent wear resistance, making it a valuable material in various industrial applications.
The Composition of Tungsten Carbide
To understand whether tungsten carbide is an alloy, we must first examine its composition. Tungsten carbide is formed through a chemical reaction between tungsten metal powder and carbon powder[20]. The resulting compound has a chemical formula of WC, indicating a 1:1 ratio of tungsten to carbon atoms.
In its pure form, tungsten carbide is not an alloy but a chemical compound. However, the material commonly referred to as "tungsten carbide" in industrial applications is often a composite material that includes a binder metal, typically cobalt[11]. This composite is created through a process called sintering, where tungsten carbide powder is mixed with the binder metal and heated to high temperatures.
The Sintering Process
The sintering process is crucial in the production of tungsten carbide components. Here's a brief overview of the steps involved:
1. Mixing: Tungsten carbide powder is mixed with a binder metal, usually cobalt.
2. Pressing: The mixture is compressed into the desired shape.
3. Heating: The compressed form is heated to temperatures between 1,400°C and 1,600°C (2,550°F to 2,900°F).
4. Binding: The binder metal melts and partially dissolves the tungsten carbide grains, creating a solid, dense material.
This process results in a material that is often referred to as "cemented carbide" or "hardmetal"[16].
Properties of Tungsten Carbide
Tungsten carbide possesses a unique combination of properties that make it highly valuable in various applications:
1. Hardness: Tungsten carbide ranks between 8.5 and 9 on the Mohs hardness scale, second only to diamond[20].
2. Density: It is approximately twice as dense as steel[19].
3. Strength: Tungsten carbide has very high compressive strength, surpassing most metals and alloys[16].
4. Wear Resistance: It exhibits excellent resistance to abrasion and wear[1].
5. Thermal Properties: Tungsten carbide has good thermal conductivity and can withstand high temperatures[15].
6. Electrical Conductivity: Its electrical conductivity is comparable to that of steel[17].
Is Tungsten Carbide an Alloy?
To answer the question posed in the title, we need to consider the definition of an alloy. An alloy is typically defined as a mixture of two or more metals, or a metal combined with one or more other elements[20]. Based on this definition, pure tungsten carbide (WC) is not an alloy but a chemical compound.
However, the material commonly referred to as "tungsten carbide" in industrial applications is often a composite material that includes a binder metal. This composite material, known as cemented carbide, could be considered an alloy system. The binder metal (usually cobalt) forms a matrix in which the tungsten carbide grains are embedded, creating a material with properties that differ from those of its individual components.
Types of Tungsten Carbide Composites
There are several types of tungsten carbide composites, each with different compositions and properties:
1. Tungsten Carbide-Cobalt (WC-Co): The most common type, using cobalt as a binder.
2. Tungsten Carbide-Nickel (WC-Ni): Uses nickel as a binder, offering better corrosion resistance.
3. Tungsten Carbide-Nickel-Chromium (WC-Ni-Cr): Provides enhanced corrosion resistance.
4. Tungsten Carbide-Titanium Carbide-Cobalt (WC-TiC-Co): Offers improved wear resistance.
These composites can be tailored to meet specific requirements by adjusting the grain size of the tungsten carbide and the amount of binder metal used[11].

Applications of Tungsten Carbide
The unique properties of tungsten carbide make it suitable for a wide range of applications across various industries:
1. Cutting Tools: Tungsten carbide is extensively used in the manufacturing of cutting tools, drill bits, and mining equipment due to its hardness and wear resistance[1].
2. Wear Parts: It is used in the production of wear-resistant components for machinery in industries such as oil and gas, mining, and construction[20].
3. Jewelry: Tungsten carbide's scratch resistance and lustrous appearance make it popular in the jewelry industry, particularly for men's wedding bands[12].
4. Armor-Piercing Ammunition: Its high density and hardness make it suitable for use in armor-piercing rounds[1].
5. Medical Instruments: Tungsten carbide is used in surgical instruments due to its durability and ability to maintain a sharp edge[10].
6. Aerospace and Automotive: It is used in various components that require high wear resistance and strength[15].
Manufacturing Process
The production of tungsten carbide involves several steps:
1. Ore Processing: Tungsten ore is processed to extract tungsten oxide.
2. Reduction: The oxide is reduced to tungsten metal powder.
3. Carburization: Tungsten powder is mixed with carbon and heated to form tungsten carbide powder.
4. Mixing: The tungsten carbide powder is mixed with a binder metal.
5. Pressing: The mixture is compressed into the desired shape.
6. Sintering: The compressed form is heated to high temperatures to create the final product.
This process allows for the creation of tungsten carbide components with precise specifications[2].
Advantages and Disadvantages
Like any material, tungsten carbide has its strengths and limitations:
Advantages:
- Exceptional hardness and wear resistance
- High strength-to-weight ratio
- Excellent thermal conductivity
- Good electrical conductivity
- Resistance to corrosion and oxidation
Disadvantages:
- Brittleness (can shatter under extreme stress)
- Difficulty in machining and shaping
- Higher cost compared to some alternative materials
- Limited ductility
Understanding these characteristics is crucial for determining the suitability of tungsten carbide for specific applications.
Future Developments
Research into tungsten carbide continues to explore new possibilities for this versatile material:
1. Nanostructured Tungsten Carbide: Developing nanostructured forms of tungsten carbide to enhance its properties further.
2. Alternative Binders: Investigating new binder materials to improve specific characteristics of tungsten carbide composites.
3. Additive Manufacturing: Exploring the use of 3D printing technologies for creating complex tungsten carbide components.
4. Coatings: Developing new coating techniques to apply thin layers of tungsten carbide to various substrates.
These advancements could lead to even more applications for tungsten carbide in the future.
Environmental Considerations
As with any industrial material, there are environmental considerations associated with tungsten carbide:
1. Mining Impact: The extraction of tungsten ore can have environmental consequences.
2. Energy Consumption: The production process of tungsten carbide is energy-intensive.
3. Recycling: Efforts are being made to improve the recycling of tungsten carbide products to reduce waste and conserve resources.
4. Substitution: Research is ongoing to find more environmentally friendly alternatives for certain applications.
Balancing the benefits of tungsten carbide with its environmental impact is an ongoing challenge for the industry.
Conclusion
In conclusion, while pure tungsten carbide (WC) is a chemical compound and not an alloy, the material commonly referred to as "tungsten carbide" in industrial applications is often a composite that includes a binder metal. This composite material, known as cemented carbide, can be considered an alloy system due to the combination of tungsten carbide with a metallic binder.
The unique properties of tungsten carbide, including its exceptional hardness, wear resistance, and thermal stability, make it an invaluable material in various industries. From cutting tools and wear-resistant components to jewelry and medical instruments, tungsten carbide continues to find new applications and push the boundaries of material science.
As research and development in this field progress, we can expect to see further innovations in tungsten carbide technology, potentially leading to new applications and improved performance in existing ones. The ongoing challenge will be to balance the benefits of this remarkable material with environmental considerations and to continue exploring ways to make its production and use more sustainable.

Frequently Asked Questions
1. What is the difference between tungsten and tungsten carbide?
Tungsten is a pure metal element, while tungsten carbide is a chemical compound made of tungsten and carbon atoms. Tungsten carbide is significantly harder and more wear-resistant than pure tungsten, making it more suitable for applications requiring extreme durability[21].
2. Can tungsten carbide be recycled?
Yes, tungsten carbide can be recycled. The recycling process involves crushing used tungsten carbide parts and chemically treating them to recover the tungsten. This recycled tungsten can then be used to produce new tungsten carbide products, helping to conserve resources and reduce waste[7].
3. Is tungsten carbide magnetic?
Pure tungsten carbide is not magnetic. However, the cobalt binder used in many tungsten carbide composites is magnetic. As a result, some tungsten carbide products may exhibit slight magnetic properties due to the presence of cobalt[12].
4. How does the hardness of tungsten carbide compare to other materials?
Tungsten carbide ranks between 8.5 and 9 on the Mohs hardness scale, making it one of the hardest known materials. It is significantly harder than steel and titanium, and is second only to diamond (which has a hardness of 10 on the Mohs scale) among commonly used materials[20].
5. Is tungsten carbide safe to wear as jewelry?
Tungsten carbide is generally safe to wear as jewelry. It is hypoallergenic for most people, as it does not contain common allergens like nickel. However, some tungsten carbide jewelry may contain small amounts of cobalt or nickel as a binder, which could cause allergic reactions in sensitive individuals. It's always best to check the specific composition of the jewelry with the manufacturer or retailer[12].
Citations:
[1] https://en.wikipedia.org/wiki/Tungsten_carbide
[2] https://heegermaterials.com/blog/90_how-is-tungsten-carbide-made-.html
[3] https://www.alamy.com/stock-photo/tungsten-carbide.html
[4] https://www.industrialplating.com/materials/tungsten-carbide-coatings
[5] https://tuncomfg.com/about/faq/
[6] https://www.retopz.com/57-frequently-asked-questions-faqs-about-tungsten-carbide/
[7] https://www.reddit.com/r/askscience/comments/9whr5d/is_tungsten_carbide_an_alloy/
[8] https://www.tungstenworld.com/pages/faq
[9] https://eternaltools.com/blogs/tutorials/tungsten-carbide-an-informative-guide
[10] https://www.tungco.com/insights/blog/5-tungsten-carbide-applications/
[11] https://konecarbide.com/what-is-tungsten-carbide/
[12] https://tungstentitans.com/pages/faqs
[13] https://www.tungco.com/insights/blog/frequently-asked-questions-used-tungsten-carbide-inserts/
[14] https://www.carbide-usa.com/top-5-uses-for-tungsten-carbide/
[15] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[16] https://www.carbideprobes.com/wp-content/uploads/2019/07/TungstenCarbideDataSheet.pdf
[17] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[18] https://www.insaco.com/material/tungsten-carbide/
[19] https://www.dymetalloys.co.uk/what-is-tungsten-carbide
[20] https://www.tungco.com/insights/blog/3-common-types-tungsten-alloys-used-today/
[21] https://touchwood.biz/blogs/southafrica/what-is-the-difference-between-pure-tungsten-and-tungsten-carbide
[22] https://www.gwstoolgroup.com/understanding-the-different-types-of-carbide-in-cutting-tools/
[23] https://create.vista.com/photos/tungsten-carbide/
[24] https://stock.adobe.com/search?k=tungsten
[25] https://www.shutterstock.com/search/tungsten-carbide-tool?image_type=illustration
[26] https://stock.adobe.com/search?k=tungsten+carbide
[27] https://www.shutterstock.com/search/tungsten-metal
[28] https://www.thermalspray.com/questions-tungsten-carbide/
[29] https://www.tungstenrepublic.com/Tungsten-Carbide-Rings-FAQ.html
















