Content Menu
● Understanding Tungsten Carbide
● Physical Properties
>> Key Physical Properties of Tungsten Carbide
● Chemical Properties
● Applications of Tungsten Carbide
>> Cutting Tools
>> Mining Tools
>> Jewelry
● The Debate: Is Tungsten Carbide a Metal?
>> Characteristics of Metals vs. Tungsten Carbide
● Manufacturing Process
● Advantages of Tungsten Carbide
● Limitations of Tungsten Carbide
● Future Trends in Tungsten Carbide Applications
● Conclusion
● FAQ
>> 1. What are the main uses of tungsten carbide?
>> 2. How does tungsten carbide compare to other materials?
>> 3. Is tungsten carbide toxic?
>> 4. Can tungsten carbide be recycled?
>> 5. How is tungsten carbide manufactured?
● Citations:
Tungsten carbide is a compound that has gained significant attention in various industries due to its exceptional properties. It is often referred to simply as "carbide" in industrial contexts. This article delves into the nature of tungsten carbide, its properties, applications, and the ongoing debate regarding its classification as a metal or a ceramic.

Understanding Tungsten Carbide
What is Tungsten Carbide?
Tungsten carbide (WC) is a chemical compound composed of equal parts of tungsten (W) and carbon (C) atoms. It is not a metal in the traditional sense but rather an inorganic compound that exhibits metallic characteristics. In its raw form, tungsten carbide appears as a fine gray powder but can be processed into various shapes through sintering, making it suitable for numerous applications in industrial machinery, cutting tools, jewelry, and more.
Physical Properties
Hardness and Density
One of the most notable features of tungsten carbide is its hardness. It ranks between 9 and 9.5 on the Mohs scale, making it one of the hardest materials available, second only to diamonds. This extreme hardness allows tungsten carbide to withstand significant wear and tear, making it ideal for cutting tools and industrial applications.
In terms of density, tungsten carbide is approximately twice as dense as steel, which contributes to its effectiveness in various applications where durability is essential.
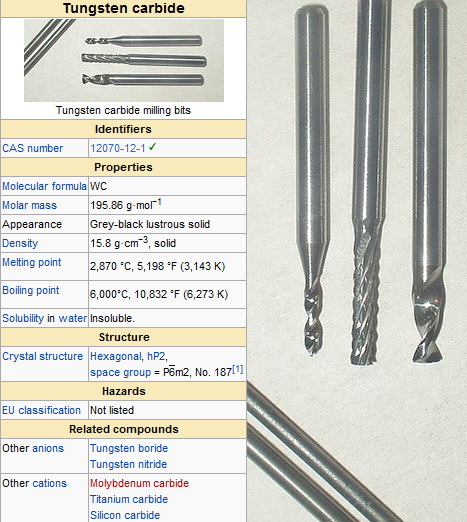
Key Physical Properties of Tungsten Carbide
- Melting Point: 2,870 °C (5,200 °F)
- Boiling Point: 6,000 °C (10,830 °F)
- Young's Modulus: Approximately 530–700 GPa
- Mohs Hardness: 9 to 9.5
- Density: Approximately 15.6 g/cm³
Chemical Properties
Tungsten carbide exhibits remarkable chemical stability. It does not oxidize at normal temperatures and maintains its properties even in harsh environments. However, it can react with certain chemicals at elevated temperatures or under specific conditions.
Applications of Tungsten Carbide
Tungsten carbide's unique properties make it suitable for a wide range of applications:
- Cutting Tools: Due to its hardness and wear resistance, tungsten carbide is extensively used in manufacturing cutting tools such as drill bits, end mills, and saw blades.
- Mining Equipment: The material's toughness makes it ideal for drill bits and other tools used in mining operations.
- Jewelry: Tungsten carbide's scratch resistance and durability have made it popular for wedding bands and other jewelry items.
- Industrial Machinery: Components made from tungsten carbide are used in various machines where high wear resistance is required.
Cutting Tools
Tungsten carbide cutting tools are essential in industries such as manufacturing and construction. These tools can maintain their sharpness longer than those made from other materials due to their hardness. The use of tungsten carbide extends beyond simple cutting; it also includes drilling and milling operations where precision is paramount.
The ability to withstand high temperatures without losing hardness makes tungsten carbide an excellent choice for high-speed machining processes.
Mining Tools
In the mining industry, tungsten carbide is utilized for drill bits that penetrate hard rock formations. The extreme conditions faced during drilling require materials that can endure high stress and abrasion. Tungsten carbide's toughness ensures that these tools last longer than their steel counterparts, reducing downtime and increasing productivity.
Jewelry
In recent years, tungsten carbide has gained popularity in the jewelry market, particularly for wedding bands. Its scratch-resistant surface ensures that rings maintain their polished look over time. Additionally, the weighty feel of tungsten carbide rings appeals to many consumers who prefer substantial jewelry.
The Debate: Is Tungsten Carbide a Metal?
The classification of tungsten carbide has been a topic of discussion among scientists and industry professionals. While it contains tungsten—a metal—it is fundamentally different from traditional metals due to its unique bonding structure.
Characteristics of Metals vs. Tungsten Carbide
| Property | Metals | Tungsten Carbide |
| Composition | Metallic elements bonded by metallic bonds | Compound of tungsten and carbon |
| Structure | Crystalline lattice with free electrons | Covalent bonds between tungsten and carbon |
| Hardness | Varies; generally less than tungsten carbide | Extremely hard (9-9.5 on Mohs scale) |
| Ductility | Generally ductile | Brittle; lacks ductility |
While metals exhibit ductility and malleability due to their free-moving electrons, tungsten carbide's structure leads to brittleness. This fundamental difference suggests that tungsten carbide should be classified as a ceramic rather than a metal.
Manufacturing Process
How Tungsten Carbide is Made
The manufacturing process of tungsten carbide involves several steps:
1. Powder Production: Tungsten powder is produced through various methods such as hydrogen reduction or carbonyl process.
2. Mixing with Carbon: The tungsten powder is mixed with carbon sources (usually graphite) in specific ratios to achieve the desired properties.
3. Sintering: The mixture is then subjected to high temperatures (around 1,400 °C to 1,600 °C) under pressure in a vacuum or inert atmosphere. This process causes the powders to bond together without melting completely.
4. Shaping: After sintering, the material can be shaped into various forms using grinding or machining techniques.
5. Finishing: Finally, the products undergo finishing processes like polishing or coating to enhance their appearance or performance.
Advantages of Tungsten Carbide
Tungsten carbide offers several advantages over traditional materials:
- Wear Resistance: Its hardness allows it to resist wear effectively.
- High Temperature Resistance: It maintains its strength at elevated temperatures.
- Corrosion Resistance: Tungsten carbide does not corrode easily compared to other metals.
- Versatility: It can be manufactured into various shapes for different applications.
Limitations of Tungsten Carbide
Despite its many advantages, tungsten carbide does have some limitations:
- Brittleness: While hard, it can be brittle under certain conditions, making it susceptible to chipping or cracking.
- Cost: The manufacturing process can be more expensive than producing traditional metals.
- Machining Difficulty: Due to its hardness, machining tungsten carbide requires specialized equipment.
Future Trends in Tungsten Carbide Applications
As technology advances, new applications for tungsten carbide are emerging:
- 3D Printing: Research into additive manufacturing techniques using tungsten carbide could revolutionize how components are produced.
- Biomedical Applications: The biocompatibility of tungsten carbide opens doors for potential use in medical implants and devices.
- Nanotechnology: Exploring nanoscale applications may lead to enhanced properties or novel uses in electronics or coatings.
Conclusion
In summary, while tungsten carbide contains metallic elements and possesses some metallic characteristics such as high density and electrical conductivity, it is fundamentally different from traditional metals due to its unique bonding structure and properties. As such, it is more accurately classified as a ceramic compound rather than a metal.
The versatility of tungsten carbide continues to make it an invaluable material across various industries—from cutting tools to jewelry—demonstrating that its unique properties can meet diverse needs effectively.

FAQ
1. What are the main uses of tungsten carbide?
Tungsten carbide is primarily used in cutting tools, mining equipment, jewelry, and industrial machinery due to its hardness and wear resistance.
2. How does tungsten carbide compare to other materials?
Tungsten carbide is significantly harder than most metals and ceramics, ranking just below diamond on the Mohs scale.
3. Is tungsten carbide toxic?
Tungsten carbide itself is not toxic; however, when processed or ground into fine particles, safety precautions should be taken to avoid inhalation or skin contact.
4. Can tungsten carbide be recycled?
Yes, tungsten carbide can be recycled from worn-out tools and components, making it an environmentally friendly choice in many applications.
5. How is tungsten carbide manufactured?
Tungsten carbide is typically produced through powder metallurgy processes involving mixing tungsten powder with carbon sources and sintering at high temperatures.
Citations:
[1] https://en.wikipedia.org/wiki/Tungsten_carbide
[2] https://www.allied-material.co.jp/en/techinfo/tungsten_carbide/features.html
[3] https://grafhartmetall.com/en/what-is-the-difference-between-ceramics-and-tungsten-carbide/
[4] https://create.vista.com/photos/tungsten-carbide/
[5] https://rrcarbide.com/where-does-scrap-carbide-comes-from/
[6] https://www.retopz.com/57-frequently-asked-questions-faqs-about-tungsten-carbide/
[7] https://www.thermalspray.com/questions-tungsten-carbide/
[8] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[9] https://www.dymetalloys.co.uk/what-is-tungsten-carbide
[10] https://www.totalmateria.com/en-us/articles/tungsten-carbide-metals-1/
[11] https://www.alamy.com/stock-photo/tungsten-carbide.html
[12] https://www.alamy.com/stock-photo/tungsten-carbide-tool.html
[13] https://eternaltools.com/blogs/tutorials/tungsten-carbide-an-informative-guide
[14] https://www.reddit.com/r/metallurgy/comments/ub4dg9/question_about_tungsten_carbide_toxicity/
[15] https://www.reddit.com/r/explainlikeimfive/comments/18ppmzu/eli5_the_difference_between_a_metal_alloy_and_a/
[16] https://eternaltools.com/blogs/tutorials/tungsten-carbide-an-informative-guide
[17] https://www.britannica.com/science/tungsten-carbide
[18] https://hanoverjewelers.com/blogs/education/tungsten-carbide-vs-ceramic-rings-whats-the-difference
[19] https://generalcarbide.com/pdf/General-Carbide-Designers-Guide-Tungsten-Carbide.pdf
[20] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[21] https://www.coorstek.com/en/materials/tungsten-carbide/
[22] https://nanopartikel.info/en/knowledge/materials/tungsten-carbide/
[23] https://www.vedantu.com/chemistry/tungsten-carbide
[24] https://www.reddit.com/r/askscience/comments/9whr5d/is_tungsten_carbide_an_alloy/
[25] https://pubchem.ncbi.nlm.nih.gov/compound/tungsten%20carbide
[26] https://todaysmachiningworld.com/magazine/how-it-works-making-tungsten-carbide-cutting-tools/
[27] https://periodictable.com/Elements/074/pictures.html
[28] https://stock.adobe.com/search?k=carbide
[29] https://www.gwstoolgroup.com/understanding-the-different-types-of-carbide-in-cutting-tools/
[30] https://stock.adobe.com/search?k=tungsten+carbide
[31] https://www.alamy.com/tungsten-carbide-inserts-image8698841.html
[32] https://www.freepik.com/free-photos-vectors/tungsten-carbide/27
[33] https://www.hydrocarbide.com/products/intricate-shapes/
[34] https://www.dreamstime.com/photos-images/tungsten-carbide.html
[35] https://www.alamy.com/tungsten-carbide-inserts-image8698850.html
[36] https://en.wikipedia.org/wiki/Tungsten_carbide
[37] https://tuncomfg.com/about/faq/
[38] https://consolidatedresources.com/blog/10-facts-about-tungsten-carbide/
[39] http://www.carbidetechnologies.com/faqs/
















