Content Menu
● Understanding Tungsten Carbide: The Basics
● Tungsten and Carbon on the Periodic Table
● Chemical Structure and Composition
>> Atomic Bonding and Crystal Structure
● Physical and Chemical Properties
>> Physical Properties
>> Chemical Properties
>> Mechanical Properties
● How Is Tungsten Carbide Made?
>> Powder Metallurgy Process
>> Advanced Manufacturing Techniques
● Tungsten Carbide vs. Tungsten: A Comparison
● Industrial and Everyday Applications
>> Cutting Tools and Machining
>> Wear-Resistant Components
>> Jewelry
>> Aerospace and Defense
>> Medical and Dental Uses
>> Sports and Recreation
● Why Is Tungsten Carbide So Hard?
>> Role of the Binder
>> Comparison to Other Hard Materials
● Manufacturing Process: From Powder to Product
>> Quality Control and Testing
● Key Properties at a Glance
● Tungsten Carbide in Modern Industry
>> Environmental Impact and Recycling
>> Future Developments
● Conclusion
● Frequently Asked Questions (FAQ)
>> 1. What is tungsten carbide and how is it different from steel?
>> 2. How is tungsten carbide manufactured?
>> 3. What are the main uses of tungsten carbide?
>> 4. Is tungsten carbide recyclable?
>> 5. Why is tungsten carbide preferred for cutting tools?
Tungsten carbide is a material that has become synonymous with industrial strength, exceptional durability, and technological innovation. But what exactly is tungsten carbide, and how does it relate to the periodic table? This comprehensive article explores the chemistry, structure, properties, and applications of tungsten carbide, offering deep insight for readers curious about its scientific foundation and real-world uses.
Understanding Tungsten Carbide: The Basics
Tungsten carbide is not a single element but a chemical compound composed of two elements: tungsten (W) and carbon (C). Its chemical formula is WC, indicating a one-to-one atomic ratio between tungsten and carbon. While both tungsten and carbon are found on the periodic table, tungsten carbide itself is a compound and does not have its own entry on the table. Instead, its properties are derived from the unique combination of these two elements.
Tungsten and Carbon on the Periodic Table
- Tungsten (W):
- Atomic Number: 74
- Group: 6
- Period: 6
- Category: Transition Metal
- Carbon (C):
- Atomic Number: 6
- Group: 14
- Period: 2
- Category: Nonmetal
Tungsten is a dense, hard metal known for its high melting point and strength, while carbon is a versatile nonmetal that forms the backbone of organic chemistry. When these two elements bond, they form tungsten carbide, a material with properties that far exceed those of its individual components.
Chemical Structure and Composition
Tungsten carbide consists of equal parts tungsten and carbon atoms, arranged in a hexagonal crystal lattice. This highly ordered structure is responsible for the compound's remarkable hardness and strength. The most common industrial form of tungsten carbide contains approximately 94% tungsten and 6% carbon by weight, though the exact ratio can be adjusted for specific applications.
Atomic Bonding and Crystal Structure
The crystal structure of tungsten carbide is a key factor in its mechanical properties. The strong covalent bonds between tungsten and carbon atoms create a rigid, tightly packed lattice. This hexagonal arrangement is similar to that of other hard materials, such as boron nitride, and is what gives tungsten carbide its impressive resistance to deformation and wear. The unique arrangement of atoms allows for minimal movement within the lattice, resulting in a material that is both tough and brittle-a rare combination in metallurgy.
Physical and Chemical Properties
Physical Properties
- Density: About 15.6 g/cm³, making it nearly as dense as gold.
- Hardness: 8.5–9 on the Mohs scale, second only to diamond.
- Melting Point: Approximately 2,870°C.
- Thermal Conductivity: 110 W/(m·K).
- Young's Modulus: 530–700 GPa, about twice that of steel.
- Color: Fine gray powder in its raw form; metallic luster when sintered.
Chemical Properties
- Stability: Highly stable and resistant to oxidation at room temperature.
- Corrosion Resistance: Resistant to most acids except a mixture of hydrofluoric and nitric acids.
- Reactivity: Reacts with chlorine above 400°C and with fluorine at room temperature.
Mechanical Properties
Tungsten carbide is renowned for its combination of hardness and toughness. While it is incredibly hard, it is also brittle, meaning it can fracture under extreme impact. However, when combined with a metallic binder such as cobalt, its toughness increases, making it suitable for use in cutting and drilling tools.
How Is Tungsten Carbide Made?
The production of tungsten carbide involves several steps:
1. Extraction of Tungsten: Tungsten is obtained from ores like scheelite and wolframite.
2. Preparation of Tungsten Powder: The ore is refined to produce pure tungsten powder.
3. Carburization: Tungsten powder is mixed with carbon (usually graphite) and heated to high temperatures (1,400–2,000°C) to form tungsten carbide powder.
4. Sintering: The powder is pressed and heated with a binder (commonly cobalt) to form solid shapes with enhanced toughness.
Powder Metallurgy Process
The powder metallurgy process is critical to achieving the desired properties of tungsten carbide. By carefully controlling the size and distribution of the powder particles, manufacturers can produce materials with specific hardness, toughness, and wear resistance. The addition of a binder, typically cobalt, helps to hold the tungsten carbide grains together, improving the material's toughness without significantly reducing its hardness.
Advanced Manufacturing Techniques
Modern advancements in manufacturing have led to the development of ultra-fine grain tungsten carbide, which offers even greater hardness and wear resistance. Techniques such as hot isostatic pressing and spark plasma sintering allow for precise control over the microstructure, enabling the production of components with exceptional performance characteristics.
Tungsten Carbide vs. Tungsten: A Comparison
| Property | Tungsten (W) | Tungsten Carbide (WC) |
| Atomic Number | 74 | Compound (W + C) |
| Density (g/cm³) | 19.3 | 15.6 |
| Melting Point (°C) | 3,422 | 2,870 |
| Hardness (Mohs) | 7.5 | 8.5–9 |
| Structure | Body-centered cubic | Hexagonal |
| Main Use | Filaments, alloys | Cutting tools, wear parts |
Industrial and Everyday Applications
Cutting Tools and Machining
Tungsten carbide's extreme hardness makes it ideal for manufacturing cutting tools such as drill bits, milling cutters, saw blades, and taps. These tools can cut through steel, titanium, and other hard materials with ease, maintaining sharpness far longer than conventional steel tools. In the world of manufacturing, the use of tungsten carbide tools has led to increased efficiency, reduced downtime, and improved product quality.
Wear-Resistant Components
Industries such as mining, oil and gas, and manufacturing utilize tungsten carbide for components exposed to high wear and abrasion. Examples include drill bits, pump seals, valve seats, and nozzles. The material's ability to withstand harsh environments means that equipment lasts longer and requires less frequent replacement, resulting in significant cost savings.
Jewelry
Tungsten carbide's metallic luster, resistance to scratching, and hypoallergenic properties have made it a popular choice for rings and other jewelry, offering a modern alternative to precious metals. Tungsten carbide rings are particularly valued for their durability and ability to maintain a polished finish over time.
Aerospace and Defense
The aerospace industry uses tungsten carbide coatings to protect turbine blades and other critical components from erosion and wear, ensuring reliability in extreme environments. In defense, tungsten carbide is used in armor-piercing ammunition and protective armor due to its density and hardness.
Medical and Dental Uses
Tungsten carbide is also used in the medical field for surgical instruments, dental drills, and prosthetic devices. Its biocompatibility and resistance to corrosion make it an ideal material for applications where precision and reliability are paramount.
Sports and Recreation
In sports, tungsten carbide is used to make tips for trekking poles, darts, and fishing weights. Its density and hardness provide superior performance compared to traditional materials.
Why Is Tungsten Carbide So Hard?
The secret to tungsten carbide's hardness lies in its atomic structure. The strong covalent bonds between tungsten and carbon atoms create a rigid, tightly packed lattice. This structure resists deformation and abrasion, allowing tungsten carbide to maintain its shape and sharpness under intense pressure and heat.
Role of the Binder
While tungsten carbide is extremely hard, it is also brittle. The addition of a binder, such as cobalt, helps to improve the toughness of the material. The binder acts as a cushion between the hard tungsten carbide grains, absorbing impact and preventing cracks from propagating through the material.
Comparison to Other Hard Materials
Tungsten carbide is often compared to diamond and ceramics in terms of hardness. While diamond is the hardest known material, tungsten carbide offers a unique balance of hardness and toughness, making it more suitable for many industrial applications.
Manufacturing Process: From Powder to Product
1. Mixing: Tungsten carbide powder is blended with a metallic binder (usually cobalt).
2. Pressing: The mixture is pressed into molds to form the desired shape.
3. Sintering: The pressed shapes are heated in a furnace, causing the binder to melt and fuse the particles together.
4. Finishing: The sintered parts are ground and polished to achieve precise dimensions and surface finish.
Quality Control and Testing
After manufacturing, tungsten carbide products undergo rigorous quality control and testing. Hardness, density, and microstructure are evaluated to ensure that each component meets the required specifications. Non-destructive testing methods, such as ultrasonic inspection and X-ray analysis, are used to detect internal flaws and ensure product reliability.
Key Properties at a Glance
- Exceptional Hardness: Nearly as hard as diamond.
- High Density: Provides stability and resistance to deformation.
- Thermal Stability: Maintains strength at high temperatures.
- Corrosion Resistance: Withstands harsh chemical environments.
- Wear Resistance: Prolongs the life of tools and components.
Tungsten Carbide in Modern Industry
Tungsten carbide's unique combination of properties has revolutionized manufacturing and engineering. It enables faster machining speeds, reduces downtime due to tool wear, and allows for the production of high-precision parts in demanding environments. Its versatility extends from heavy industry to consumer products, making it one of the most valuable materials in modern technology.
Environmental Impact and Recycling
The production of tungsten carbide requires significant energy and resources, but the material's durability and recyclability help offset its environmental impact. Used tungsten carbide tools and components can be collected, processed, and recycled to recover valuable tungsten and cobalt. This not only conserves resources but also reduces the need for mining and lowers the environmental footprint of manufacturing.
Future Developments
Research continues into new formulations and manufacturing techniques for tungsten carbide. Nanostructured tungsten carbide, for example, offers even greater hardness and wear resistance, opening up new possibilities for advanced applications. The integration of tungsten carbide with other materials, such as ceramics and composites, is also being explored to create hybrid materials with tailored properties.
Conclusion
Tungsten carbide is a remarkable compound born from the union of tungsten and carbon-two elements with distinct properties on the periodic table. Its unique crystal structure and exceptional physical characteristics have made it a cornerstone of modern industry, from manufacturing and mining to jewelry and aerospace. Understanding tungsten carbide's chemistry, properties, and applications reveals why it remains one of the most sought-after materials for demanding environments.
As technology advances, the role of tungsten carbide is only set to grow, with ongoing research promising even greater performance and sustainability. Whether in the tip of a drill bit, the band of a wedding ring, or the blades of a jet engine, tungsten carbide continues to shape the world around us with its unparalleled strength and resilience.
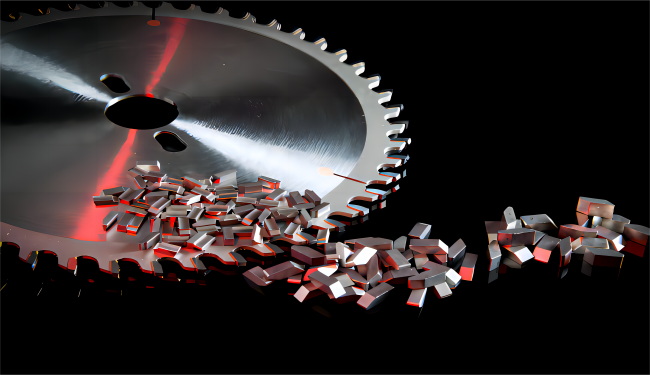
Frequently Asked Questions (FAQ)
1. What is tungsten carbide and how is it different from steel?
Tungsten carbide is a compound made from tungsten and carbon atoms in a 1:1 ratio. Unlike steel, which is an alloy of iron and carbon, tungsten carbide is much harder, more wear-resistant, and can withstand higher temperatures. This makes it ideal for applications where durability and performance are critical.
2. How is tungsten carbide manufactured?
Tungsten carbide is produced through powder metallurgy. Tungsten and carbon powders are mixed, pressed into shape, and sintered at high temperatures with a binder metal like cobalt. This process creates a dense, hard material suitable for industrial use.
3. What are the main uses of tungsten carbide?
Tungsten carbide is used in cutting tools, mining equipment, wear-resistant parts, jewelry, and aerospace components. Its hardness and durability make it indispensable in industries that require materials to withstand extreme conditions.
4. Is tungsten carbide recyclable?
Yes, tungsten carbide can be recycled. Worn-out tools and scrap materials are collected, processed, and reused to manufacture new products, helping conserve resources and reduce environmental impact.
5. Why is tungsten carbide preferred for cutting tools?
Tungsten carbide's extreme hardness, resistance to wear, and ability to maintain sharpness under high-stress conditions make it the material of choice for cutting tools. It enables faster cutting speeds, longer tool life, and improved efficiency in manufacturing.
















