Content Menu
● Introduction to Tungsten Carbide
>> Historical Context
● Chemical Composition and Structure
● Properties of Tungsten Carbide
>> Physical and Mechanical Properties
>> Chemical Properties
● What Constitutes a Salt?
>> Comparison with Diamond
● Industrial Applications
>> 1. Cutting and Machining Tools
>> 2. Wear-Resistant Components
>> 3. Aerospace and Defense
>> 4. Consumer Goods
● Advanced and Emerging Applications
>> 1. Nuclear Reactors
>> 2. Medical Devices
>> 3. Additive Manufacturing
>> 4. Renewable Energy
● Production Process: From Ore to Composite
● Challenges and Limitations
● Market Trends and Future Outlook
● Health and Safety Considerations
● Conclusion
● FAQs
>> 1. Why isn't tungsten carbide classified as a salt?
>> 2. Can tungsten carbide rust or corrode?
>> 3. How does tungsten carbide compare to titanium?
>> 4. Is tungsten carbide recyclable?
>> 5. What industries rely most on tungsten carbide?
● Citations:
Tungsten carbide, with the chemical formula WC, is a compound made of tungsten and carbon atoms. Renowned for its exceptional hardness, wear resistance, and high melting point, it has become indispensable in industries ranging from manufacturing to aerospace. However, the question of whether tungsten carbide qualifies as a salt requires a deep dive into its chemical structure, formation process, and properties.

Introduction to Tungsten Carbide
Tungsten carbide is not a traditional metal alloy but a composite material produced through powder metallurgy. In this process, fine grains of tungsten carbide are combined with a metallic binder—typically cobalt or nickel—and sintered at temperatures exceeding 1,400°C to form a dense, ultra-hard material. Its Young's modulus (530–700 GPa) surpasses that of steel by a factor of three, making it one of the stiffest materials available for industrial use.
Historical Context
The discovery of tungsten carbide dates back to the late 19th century, but its commercial viability was realized in the 1920s when German scientists developed methods to sinter tungsten carbide with cobalt binders. This innovation revolutionized cutting tools, replacing high-speed steel in applications requiring extreme durability. By the 1950s, tungsten carbide became a cornerstone of the machining and mining industries.
Chemical Composition and Structure
Tungsten carbide consists of equal parts tungsten and carbon atoms arranged in a hexagonal crystal lattice. Industrial grades often include 94% tungsten carbide and 6% cobalt by weight, though formulations vary depending on the application. For example:
- High-Cobalt Blends (10–20%): Improve toughness for impact-resistant tools.
- Nickel-Based Binders: Enhance corrosion resistance for marine or chemical environments.
Unlike salts, which are ionic compounds, tungsten carbide features covalent bonds between tungsten and carbon atoms. This covalent bonding contributes to its extreme hardness and thermal stability.
Properties of Tungsten Carbide
Physical and Mechanical Properties
1. Hardness:
- Vickers hardness: 1,500–2,200 HV, rivaling corundum (α-Al2O3) and approaching diamond (10,000 HV).
- Retains hardness even at temperatures up to 1,000°C.
2. Density:
- 15.6 g/cm³, comparable to gold, which enhances its vibration-damping capabilities.
3. Thermal Conductivity:
- 110 W/m·K, enabling efficient heat dissipation in cutting tools.
Chemical Properties
- Corrosion Resistance: Resistant to acids, alkalis, and organic solvents, though it reacts with hydrofluoric acid and fluorine gas.
- Oxidation Resistance: Stable in air up to 600°C; oxidizes to tungsten trioxide (WO3) at higher temperatures.
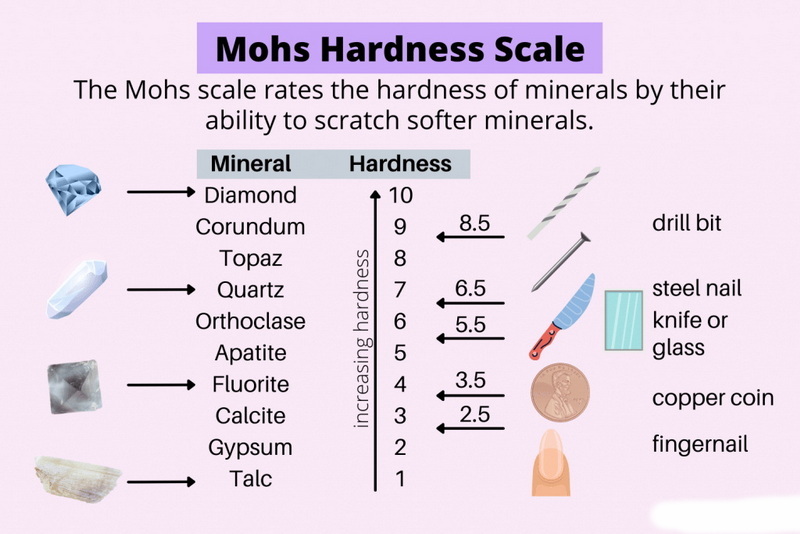
What Constitutes a Salt?
Salts are ionic compounds formed through the neutralization of acids and bases. Key characteristics include:
- Ionic Bonding: Electrostatic attraction between positively charged cations (e.g., Na⁺) and negatively charged anions (e.g., Cl⁻).
- Solubility: Many salts dissolve in water, releasing ions.
- Conductivity: Salts conduct electricity in molten or dissolved states.
Examples include sodium chloride (NaCl) and calcium sulfate (CaSO4). Tungsten carbide, in contrast, lacks ionic bonding and insolubility in water, disqualifying it as a salt.
| Property | Tungsten Carbide | Alumina Ceramic |
| Hardness (HV) | 1,500–2,200 | 1,500–1,800 |
| Fracture Toughness | 8–10 MPa√m | 3–4 MPa√m |
| Thermal Conductivity | 110 W/m·K | 30 W/m·K |
Comparison with Diamond
While diamond is harder (10,000 HV), tungsten carbide offers better toughness and cost-effectiveness for industrial tools.
Industrial Applications
1. Cutting and Machining Tools
- Drill Bits: Tungsten carbide tips extend tool life in rock drilling and metalworking.
- Inserts for Lathes: Used in CNC machining to cut stainless steel and titanium alloys.
2. Wear-Resistant Components
- Valve Seals: Withstand abrasive fluids in oil and gas pipelines.
- Excavator Teeth: Reduce wear in mining operations.
3. Aerospace and Defense
- Rocket Nozzles: Tolerate extreme temperatures and erosive exhaust gases.
- Armor-Piercing Ammunition: Utilizes dense tungsten carbide cores.
4. Consumer Goods
- Jewelry: Hypoallergenic rings and watches prized for scratch resistance.
- Sports Equipment: Golf club inserts and bicycle pedals.
Advanced and Emerging Applications
1. Nuclear Reactors
Tungsten carbide's high neutron absorption cross-section makes it suitable for control rods and shielding components.
2. Medical Devices
- Surgical Tools: Scalpel blades and bone drills benefit from sharpness and sterility.
- Dental Implants: Biocompatible coatings improve longevity.
3. Additive Manufacturing
3D-printed tungsten carbide parts are being tested for custom tooling and aerospace components.
4. Renewable Energy
- Wind Turbine Bearings: Reduce maintenance in harsh environments.
- Hydrogen Fuel Cells: Coatings protect bipolar plates from corrosion.
Production Process: From Ore to Composite
1. Ore Refining: Tungsten is extracted from wolframite or scheelite ores.
2. Carburization: Tungsten powder reacts with carbon at 1,400–2,000°C to form WC.
3. Milling: WC particles are ground to submicron sizes.
4. Mixing with Binder: Cobalt or nickel is added to the WC powder.
5. Pressing and Sintering: Compressed into shapes and sintered in vacuum furnaces.
Challenges and Limitations
1. Brittleness: Prone to chipping under sudden impact, limiting use in dynamic loads.
2. Cost: Raw tungsten prices fluctuate due to geopolitical factors (e.g., 80% of reserves are in China).
3. Environmental Impact: Mining releases heavy metals, necessitating strict waste management.
Market Trends and Future Outlook
The global tungsten carbide market is projected to grow at 6.2% CAGR from 2024 to 2030, driven by:
- Automotive Electrification: Demand for machining electric vehicle components.
- Sustainable Practices: Recycling initiatives recover up to 95% of tungsten from scrap.
- Nanostructured WC: Nanoparticles enhance hardness and reduce binder requirements.
Health and Safety Considerations
- Dust Exposure: Inhalation of WC-Co powders may cause lung fibrosis; workplaces require HEPA filtration.
- Machining Precautions: Use coolant to suppress dust during grinding.
Conclusion
Tungsten carbide is unequivocally not a salt but a covalent ceramic-metal composite with unparalleled mechanical properties. Its applications span from everyday tools to cutting-edge technologies, and ongoing research continues to unlock new possibilities. While challenges like brittleness and environmental concerns persist, advancements in recycling and nanotechnology promise a sustainable future for this remarkable material.

FAQs
1. Why isn't tungsten carbide classified as a salt?
Tungsten carbide lacks ionic bonding and is insoluble in water—key traits of salts. It forms covalent bonds instead.
2. Can tungsten carbide rust or corrode?
It resists corrosion in most environments but oxidizes at high temperatures (>600°C) and reacts with strong acids like HF.
3. How does tungsten carbide compare to titanium?
Tungsten carbide is harder and denser but less ductile. Titanium is lighter and more biocompatible for implants.
4. Is tungsten carbide recyclable?
Yes, scrap WC is crushed and reprocessed into new powder, reducing reliance on virgin tungsten.
5. What industries rely most on tungsten carbide?
Mining (35%), manufacturing (30%), and aerospace (15%) are the largest consumers globally.
Citations:
[1] https://en.wikipedia.org/wiki/Tungsten_carbide
[2] https://www.linde-amt.com/resource-library/articles/tungsten-carbide
[3] https://carbideprocessors.com/pages/carbide-parts/tungsten-carbide-properties.html
[4] https://www.istockphoto.com/photos/tungsten-carbide
[5] https://www.alamy.com/stock-photo/tungsten-carbide.html
[6] https://www.freepik.com/free-photos-vectors/tungsten-carbide
[7] http://www.chinatungsten.com/Tungsten-Carbide/Properties-of-Tungsten-Carbide.html
[8] https://en.wikipedia.org/wiki/Tungsten
[9] https://hpvchemicals.oecd.org/ui/handler.axd?id=ed1c76bf-dad9-4baa-8d1b-70fed7f92862
[10] https://www.tungco.com/insights/blog/5-tungsten-carbide-applications/
[11] https://www.britannica.com/science/tungsten-carbide
[12] https://www.carbide-usa.com/top-5-uses-for-tungsten-carbide/
[13] https://periodictable.com/Elements/074/pictures.html
[14] http://www.tungsten-carbide.com.cn
[15] http://picture.chinatungsten.com/list-18.html
















